Regulation of Gut Dysbiosis and Its Potential Effects on Neurodegenerative Diseases
Author: Abigail Bondurant
Neuroscience In Review
Introduction
Diseases of the central nervous system (CNS), such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) ultimately result in the death of neurons as the diseases progress. Alzheimer’s disease and Parkinson’s disease are neurodegenerative disorders that develop when neurons in the CNS begin to function abnormally before eventually dying. Abnormal functions include disrupted firing patterns, altered synaptic connections, or excess production of neurotransmitters or proteins (Hutchins & Barger, 1998; Conners & Regehr, 2004). Complete loss of function can result from damage to the cell during a head injury or stroke, spinal cord injury, or infection, as well as the progression of AD and PD. The damage sustained during any of these factors, as previously mentioned, can cause a loss of neuronal connections, or spontaneous genetic mutations that result in excess production or decreased production of neurotransmitters (Hutchins & Barger, 1998). These factors can also cause neuronal cell death by producing free radicals, proteins accumulation, chronic inflammation, and necrosis (Hutchins & Barger, 1998).
Alzheimer’s disease and Parkinson’s disease are currently the most common neurodegenerative disorders (National Institute of Environmental Health Sciences) worldwide. Alzheimer’s disease is the most prevalent form of dementia (National Institute on Aging, 2021). According to the CDC, there was about 5.8 million people in the United States that had been diagnosed with AD (Centers for Disease Control and Prevention, 2020). PD is the second most common neurodegenerative disorder and around 1 million people are thought to be living with Parkinson’s disease in the U.S. in 2020 (Naqvi, 2018 (updated in 2020)).
These diseases are debilitating because they can cause deficits in motor function, memory, learning, and other physiological processes such as breathing. AD is characterized by the accumulation of extracellular amyloid-beta (Aβ![]() ) plaques and hyperphosphorylated tau proteins that develop into neurofibrillary tangles intracellularly (Kwon & Koh, 2020). The build-up of neurofibrillary tangles and Aβ
) plaques and hyperphosphorylated tau proteins that develop into neurofibrillary tangles intracellularly (Kwon & Koh, 2020). The build-up of neurofibrillary tangles and Aβ![]() plaques become neurotoxic, which causes neurons to lose function before eventually dying. The neuronal cell death leads to deficits in cognitive functioning, such as learning, memory and the formation coherent sentences when speaking (National Institute on Aging, 2021). AD can also cause changes in behavior, for example increased aggression and anxiety, wandering and agitation (National Institute on Aging, 2021). PD is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta in the brain and the accumulation of Lewy bodies through aggregated α
plaques become neurotoxic, which causes neurons to lose function before eventually dying. The neuronal cell death leads to deficits in cognitive functioning, such as learning, memory and the formation coherent sentences when speaking (National Institute on Aging, 2021). AD can also cause changes in behavior, for example increased aggression and anxiety, wandering and agitation (National Institute on Aging, 2021). PD is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta in the brain and the accumulation of Lewy bodies through aggregated α![]() -synuclein protein (Kwon & Koh, 2020; Lin et al, 2019). Symptoms of PD include slowed movements, also known bradykinesia, a decrease in the ability to perform automatic movements such as blinking or arms swinging when walking, speech changes, and tremors (Mayo Clinic, 2022).
-synuclein protein (Kwon & Koh, 2020; Lin et al, 2019). Symptoms of PD include slowed movements, also known bradykinesia, a decrease in the ability to perform automatic movements such as blinking or arms swinging when walking, speech changes, and tremors (Mayo Clinic, 2022).
Many symptoms are mild in the beginning stages of these diseases and become progressively worse as more neurons lose function and die. There are no cures or effective ways to prevent or slow the progression of AD or PD, and the risk for developing either of these diseases increases as a person ages. It has been determined that genes contribute to the development of a certain disease by increasing the susceptibility to the disease (National Institute of Environmental Health Sciences). Some examples of genetic factors for AD and PD include APOE e4, and LRRK22, PINK1, or SNCA, respectively. In addition to the genetic factors, environmental factors also play a role in the development and the severity of AD and PD (National Institute of Environmental Health Sciences).
In this review, I’ll be focusing on the gut-brain axis, more specifically how dysregulation of our microbiota can play a role in the progression of AD and PD by exacerbating neuroinflammation.
AD and PD are often accompanied by neuroinflammation, which is an inflammatory response within the CNS. This kind of inflammation can be caused by an injury, changes to the composition of the gut microbiota, a virus, and an infection, amongst other factors. Neuroinflammation can be beneficial because it provides protection when there is an injury to the brain or body, or during an infection (Lyman et al, 2014). It is only when the inflammatory response becomes chronic that it is damaging to the CNS (DiSabato et al, 2016; Kempuraj et al, 2016). Chronic neuroinflammation can exacerbate disease progression and cause neuronal cell death (DiSabato et al, 2016; Kempuraj et al, 2016). Neuroinflammation can also have a negative effect on OR be negatively impacted by the gut microbiota the growth of harmful bacteria.
Neuroinflammation and its Role in Neurodegenerative Diseases
Inflammation, whether it is central (affecting the brain and spinal cord) or peripheral (affecting the limbs and trunk), is the response that is triggered by our immune system as a form of protection against pathogens, infections, or injury (Lyman et al, 2014). The inflammatory response can either be acute or chronic. Acute inflammation is how our immune system is able to get control over what has triggered the response in the first place (the stimulus). The stimulus is removed via phagocytosis, and immune cells are recruited to the site to begin tissue regeneration, removal of cellular debris, and scar formation (Kempuraj et al, 2016; Lyman et al, 2014). This, however, does not apply to sterile inflammation. This type of response is not triggered by an external factor, but rather an intracellular one, such as cytokines released from necrotic cells that induce inflammation by activating the immune system (Chen & Nuñez, 2021).
When inflammation is prolonged, it becomes a chronic inflammatory response. Chronic inflammation can be damaging to the brain because it can result in neuronal cell death by inhibiting tissue regeneration, increasing the permeability of the blood brain barrier, and increased cytokine and chemokine production (DiSabato et al, 2016). Inflammatory mediators (cytokines) such as TNF-α![]() and interleukins (IL), are a part of the response and are essential for clearing injured of dead cells, pathogens, or toxins. But during chronic inflammation these mediators become elevated and began to accumulate. The accumulation of proinflammatory cytokines can the signal for release of more cytokines, which can cause increased neuroflammation and neurodegeneration (Kempuraj et al, 2016) (figure 1).
and interleukins (IL), are a part of the response and are essential for clearing injured of dead cells, pathogens, or toxins. But during chronic inflammation these mediators become elevated and began to accumulate. The accumulation of proinflammatory cytokines can the signal for release of more cytokines, which can cause increased neuroflammation and neurodegeneration (Kempuraj et al, 2016) (figure 1).
In the case of AD and PD, neuroinflammation is often a secondary response that can exacerbate disease progression by negatively impacting cognitive and motor function, and causing neuronal cell death (Kempuraj et al, 2016). Neuroinflammation is a secondary response because begins after the development of either disease. As the neurons become damaged and begin to loss function, microglia are activated, reactive oxygen species (ROS) are produced, and the activity of pro-inflammatory cytokines is increased (Kempuraj et al, 2016; Zhao et al, 2019).
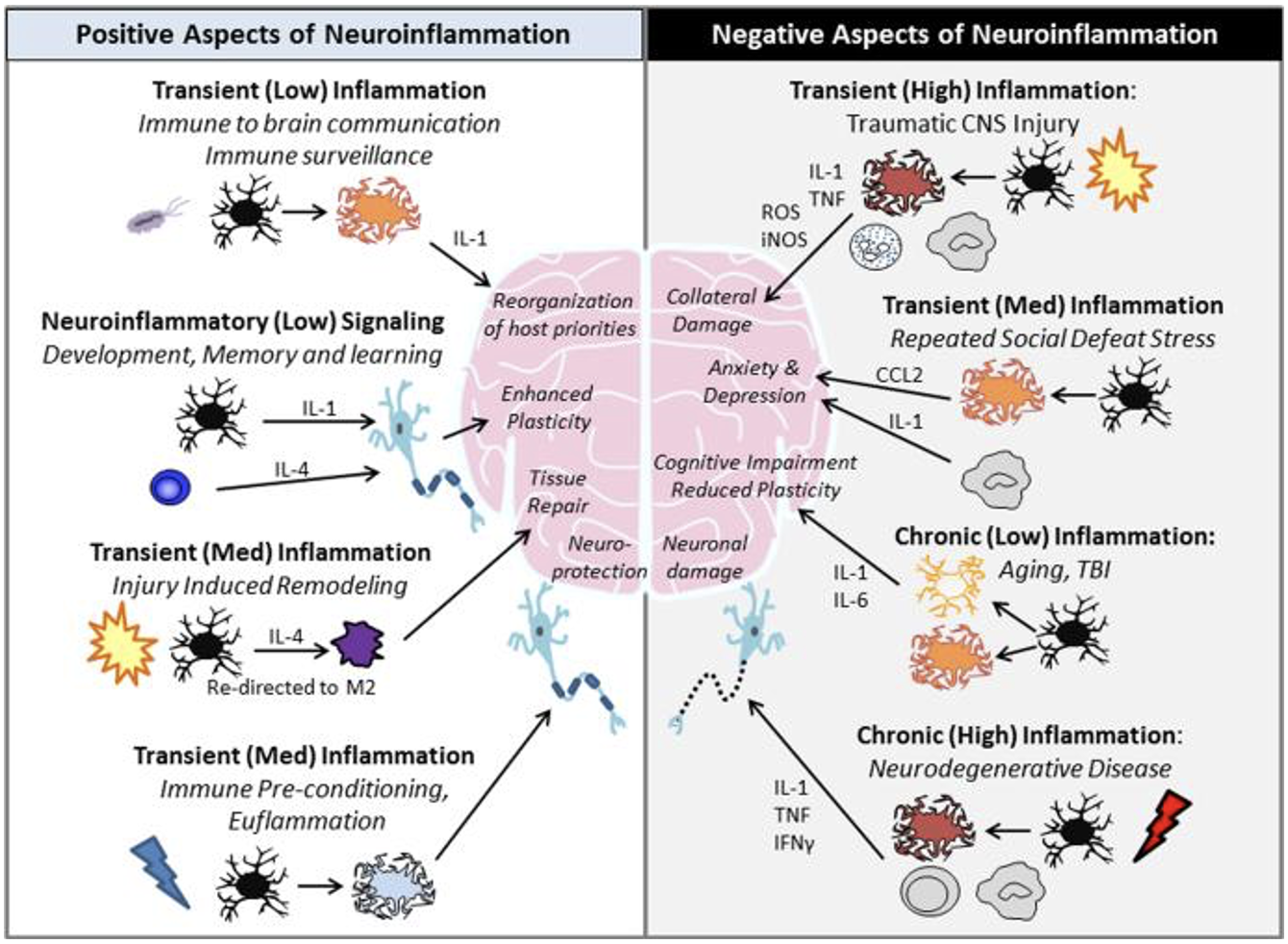
Whether the inflammatory response in acute or chronic, neuroinflammation has more than one cause. It can be caused by an injury to the spinal cord or the brain, during the contraction of a virus or infection, or even through a change of gut microbiota composition. AD and PD patients have been shown to suffer from an imbalance of our gut microbiota as well as neuroinflammation (Lin et al, 2019; Marizzoni et al, 2020).
Gut Microbiota, Dysbiosis, and How it Contributes to Neuroinflammation
The human body is home to around 100 trillion microorganisms, which is known as the human microbiota (Clemente et al, 2012). The microbiota includes bacterial, eukaryotic, viral, and archaeon organisms, but is mainly dominated by bacteria. The genes encoded by these microorganisms is known as the human microbiome (Clemente et al, 2012). The gut microbiota communicates with the CNS through what is known as the gut-brain axis and can influence the brain to release neurotransmitters, as an example (Komanduri et al, 2019).
Disruption of the composition of the gut microbiota can be caused by increased production of reactive oxygen species (which a majority are produced from the electron transport chain), aging, inflammation, diet, antibiotics, and pathogens (Komanduri et al, 2019) (figure 2).
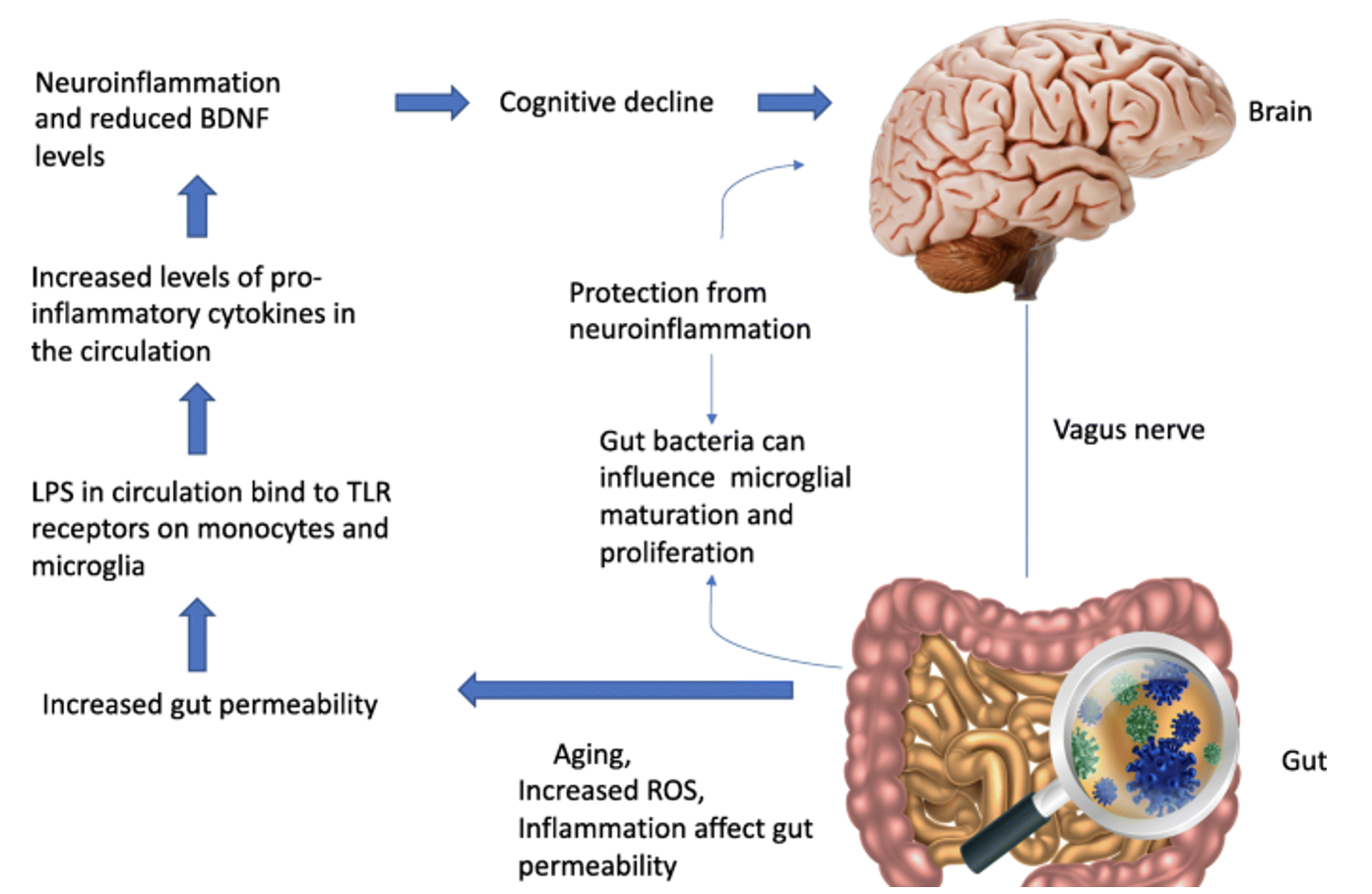
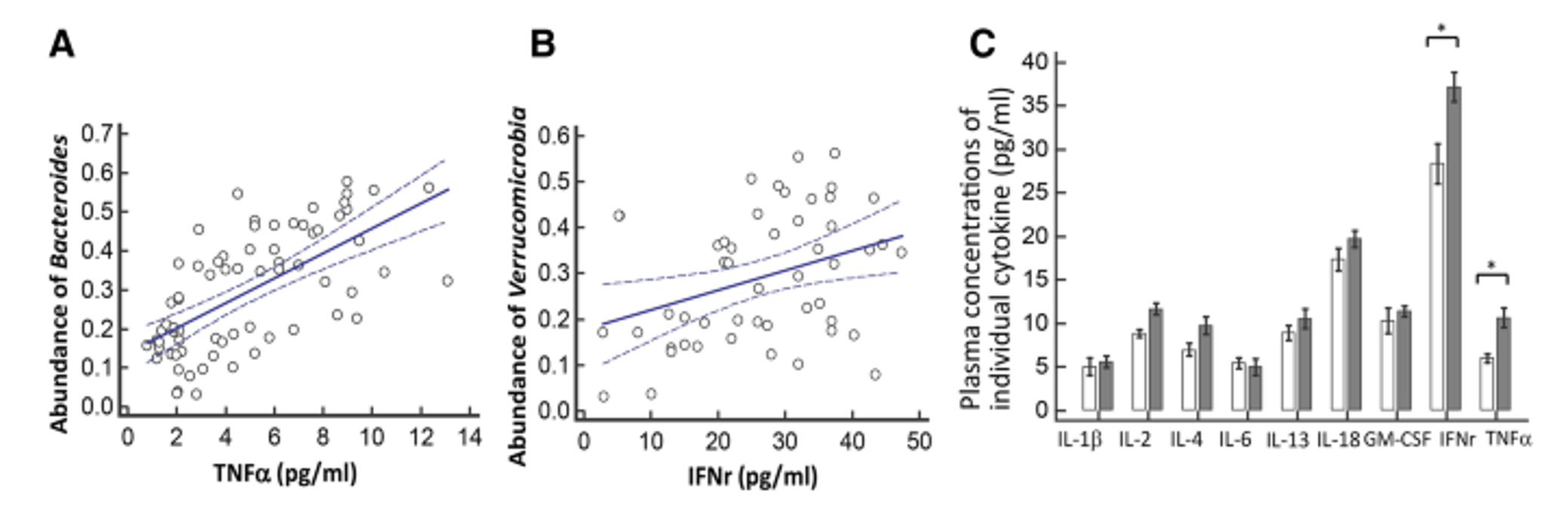
The change to microbiota composition occurs when certain bacterial strains, such as Bacteroidetes and Firmicutes, are replaced with gram negative bacteria like Bacteroides and Proteobacteria that contain lipopolysaccharides (LPS) (Komanduri et al, 2019). The bacterial product LPS has been identified as a ligand for Toll-like receptor 4 (TLR-4), which are expressed on microglia (Zhao et al, 2019). When bound to the receptor, LPS can activate microglia and stimulate these immune cells to produce pro-inflammatory cytokines (Zhao et al, 2019). In a study from 2019, the authors showed there was a correlation between certain bacterial species and increased expression of pro-inflammatory cytokines in patients diagnosed with PD that suffered from gut dysbiosis (Lin et al, 2019) (figure 3). The results showed that there was a correlation between increased plasma concentration of TNF-α and Bacteroides and between IFNr and Verrucomicrobia (Lin et al, 2019).
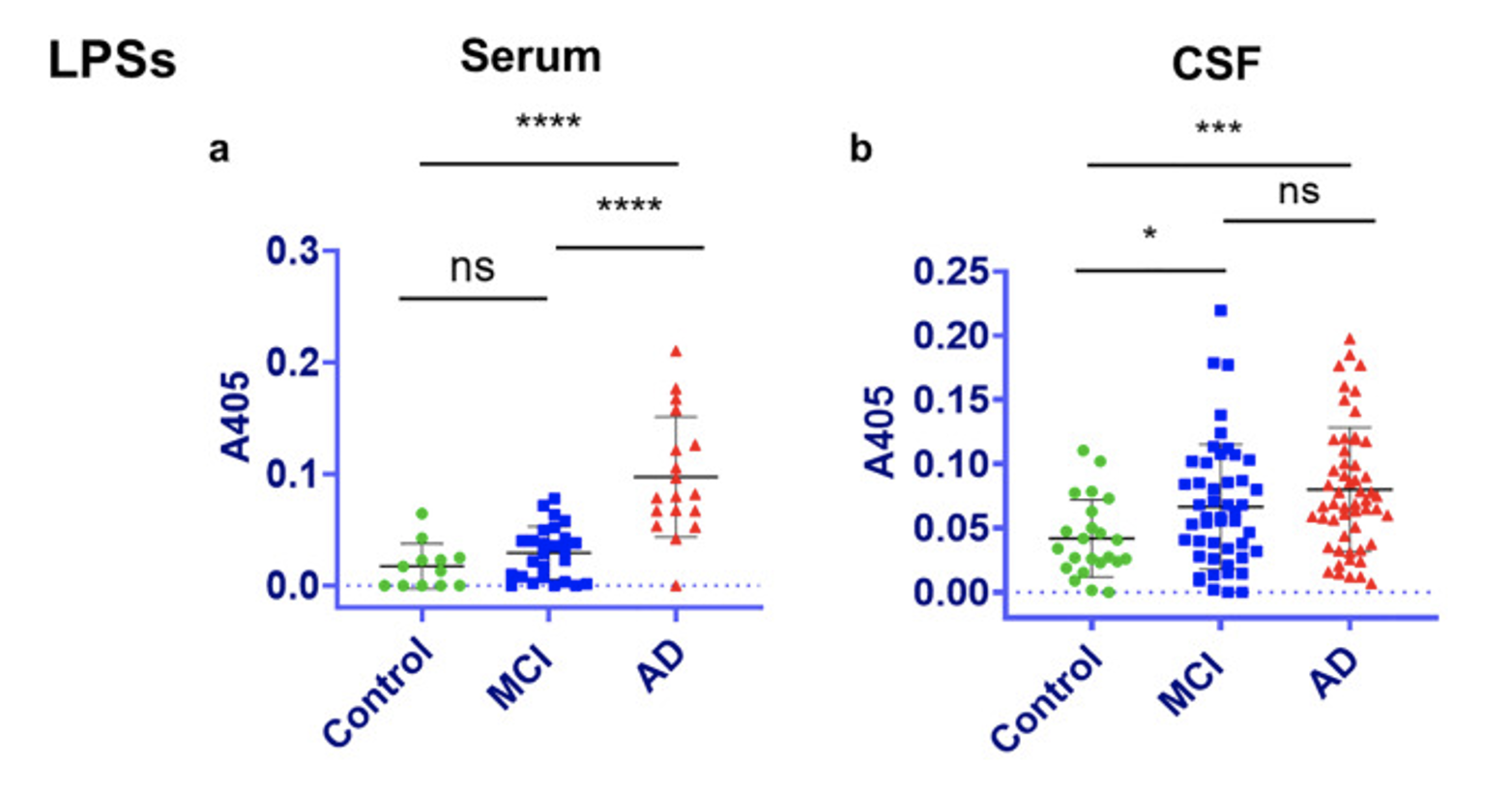
In another study, LPS was found to have increased levels in the cerebrospinal fluid (CSF) and blood serum of AD patients compared to patient with mild cognitive impairment (MCI) and healthy controls (Andreadou et al, 2021) (figure 4).
LPS is a product of gut dybiosis that is introduced to the intestinal tract through the addition of gram-negative bacteria. The addition of gram-negative bacteria increases the permeability of the intestinal tract and makes it easy for LPS to enter the blood stream and cross the blood brain barrier. Once inside the brain, LPS binds to microglia via TLR-4 and activates them, which causes a signaling cascade that increases pro-inflammatory cytokines like TNF-α![]() , IFNr, IL-6, IL-2, IL-13, and IL-1β
, IFNr, IL-6, IL-2, IL-13, and IL-1β![]() in circulation. These cytokines increase neuroinflammation and can exacerbate disease progression of AD and PD.
in circulation. These cytokines increase neuroinflammation and can exacerbate disease progression of AD and PD.
Discussion
Currently, medication for AD and PD, which are cholinesterase inhibitors and Levadopa, respectively, are aimed at reducing the severity of the symptoms associated with either disease to better the quality of life (Kempuraj et al, 2016). But one symptom that is not included in the treatments for these diseases is gut dybiosis. Regulation of gut dysbiosis could reduce the levels of LPS in circulation by replacing harmful bacteria with beneficial bacteria via fecal microbiota transplants. The reduction in LPS could, in theory, decrease the activation of microglia, which would in turn decrease the activity of pro-inflammatory cytokines. Using the gut-brain axis to target neuroinflammation could provide a new avenue for treatments of AD and PD.
But there are precautions and limitations to be aware of when discussing treatment of gut dysbiosis or neuroinflammation in AD and PD patients. Overall, there have been several studies linking gut dysbiosis to AD and PD, to neuroinflammation, or to both. But the biggest take away from those studies is that the authors used mice models for either disease. While the models are a good substitute for patients suffering from AD or PD, it is important to note that the results of the studies cannot be directly translated to humans. More information about how regulation of gut dysbiosis affects disease progression as well as symptom severity in humans, and support for using the gut-gut axis as a starting point for new treatments options of neurodegenerative disorders is needed as the topic is still relatively new.
[+] References
“Alzheimer’s Disease Fact Sheet.” (2021). National Institute on Aging, U.S. Department of Health and Human Services. https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet.
Andreadou, E.G., Katsipis, G., Tsolaki, M. & Pantazaki, A.A. (2021). Involvement and Relationship of Bacterial Lipopolysaccharides and Cyclooxygenases Levels in Alzheimer’s Disease and Mild Cognitive Impairment Patients. Journal of Neuroimmunology, vol. 357.
Chen, G. & Nuñez, G. (2010). Sterile Inflammation: Sensing and Reacting to Damage. Nat Rev Immunol (10), 826-837.
Clemente, J.C. et al. (2012). The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell, Cell Press, 15.
Conners,.B.W. & Regehr, W.G. (2004). Neuronal Firing: Does Function Follow Form? Current Biology, Cell Press, 30.
DiSabato, D. J., Quan, N., & Godbout, J. P. (2016). Neuroinflammation: The Devil is in the Details. Journal of Neurochemistry, 139 Suppl 2(Suppl 2), 136-153.
Hutchins, J.B. & Barger, S.W. (1998). Why Neurons Die: Cell Death in the Nervous System. The Anatomical Record, U.S. National Library of Medicine.
Kempuraj, D., Thangavel, R., Natteru, P. A., Selvakumar, G. P., Saeed, D., Zahoor, H., Zaheer, S., Iyer, S. S., & Zaheer, A. (2016). Neuroinflammation Induces Neurodegeneration. Journal of Neurology, Neurosurgery and Spine, 1(1), 1003.
Komanduri, M. et al. (2019). The microbiome and cognitive aging: a review of mechanisms. Psychopharmacology. 236. 10.1007/s00213-019-05231-1.
Kwon, H.S., & Koh, SH. (2020). Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes.
Lin, C.H. et al (2019). Altered Gut Microbiota and Inflammatory Cytokine Responses in patients with Parkinson’s Disease. Journal of Neuroinflammation, 129(16).
Lyman, M., Lloyd, D. G., Ji, X., Vizcaychipi, M. P., & Ma, D. (2014). Neuroinflammation: The Role and Consequences. Neuroscience Research, (14), 1-12.
Marizzoni, M, et al. (2020). Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s disease. Journal of Alzheimer’s Disease, 78(2).
Naqvi, E. (2018). Parkinson’s Disease Statistics. Parkinson’s News Today. https://parkinsonsnewstoday.com/parkinsons-disease-statistics/
“Parkinson’s Disease.” (2022). Mayo Clinic, Mayo Foundation for Medical Education and Research.
“What Is Alzheimer’s Disease?” (2020). Centers for Disease Control and Prevention, Centers for Disease Control and Prevention. https://www.cdc.gov/aging/aginginfo/alzheimers.htm.
Zhao, J. et al. (2019). Neuroinflammation Induced by Lipopolysaccharide Causes Cognitive Impairment in Mice. Sci Rep 9, 5790.
[+] Other Work By Abigail Bondurant
How Glutamatergic Proteins Influence the Maturation and Development of the Human Visual Cortex
Neuroanatomy
The Relationship Between Procedural Memory and Purkinje Cells
Neurophysiology
A new study shows that a protein, known as SHISA6, plays an essential role in the formation of procedural memory for vestibulo-ocular reflex and eye movement conditioning.