I took this new sleeping pill and nhjmjjj...... (it worked)
An Oxford research study, conducted by Kemph, Song, Talbot and Miesenböck, found evidence that the ratio of different states of NADPH had a direct effect on Shaker potassium channels which cause sleeping. This research helps shed light on the mechanisms of sleeping and could help those suffering from insomnia as well as develop better sleeping pills.
Author: Gavin Harvey
Download: [ PDF ]
Neuroanatomy
Sleep is still a process that is shrouded in mystery. Currently little is known as to why we need sleep just that when we do not get it a series of processes occur that effect metabolism, cellular repair, and cognitive function in a detrimental manner.1,2 It is known that certain neurons in the Superchiasmatic nucleus regulate the circadian rhythm in the mammalian hypothalamus3 as well as others that fire when one is asleep but remain inhibited while awake. It has been proposed that we sleep to repair damage and relieve oxidative stress accumulated while awake4 and this is a major area of study. In this study there was a relationship found between A-type currents through Shaker’s KVβ subunit (the channel that is on the neurons that seem to trigger a sleeping state) and oxidative bonding to NADPH. If we could better understand the mechanisms that influence how and why we sleep, we could determine why some individuals experience sleep related illnesses like insomnia. We could also develop medication to aid in better rest and recovery without deleterious side effects.
In this study the purpose was to find a connection between metabolism, oxidation and sleep as these processes have been linked to the processes of aging and disease.5,6 The animal used in this study was the fruit fly Drosophila because it has a small, identifiable sleep-inducing neurons7,8 which project to the dorsal Fan-shaped Body (dFB). These induce sleep when they are activated, which means they emit a constant flow of action potential called an Alpha current (IA). The mechanism is somewhat complex but, simply put, it involves two types of ion channel enzymes. Essentially during waking periods, the potassium ion channel called Sandman causes the activation of these sleep switch neurons to be irregular and harder to achieve. Then some unidentified mechanisms cause the ion channels in the cell membrane to be transported to a storage area within the cytoplasm (the inside of the cell). During this process the previously inhibited Shaker ion channels then become active (these are active during sleep). This then results in the neurons becoming active and expressing an IA that appears to activate the state of sleep in the subject.
In this experiment several different experiments were performed on the Shaker channel protein. The first focused on mutations of the subunit of Shaker called the Hyperkinetic. In this experiment it was determined that flies that did not have mutated Hyperkinetic, one that resulted in its improper oxidoreductase activity, found that flies with the mutation showed insomniac behavior while the wild-type flies without it did not. Then levels of redox histories were compared between sleep-deprived flies and a control group of rested flies. To do this the researchers labelled mitochondria of the dFB with a florescent protein that would react when oxidized by reactive oxygen species (ROS). They determined that the level of sleep deprivation correlated to an almost equal augmentation in the light detected which correlated with previous research.9
Now the primary test in this experiment was performed by introducing a flavoprotein into dFB neurons of the Drosophila. These proteins would activate when exposed to a specific blue light. This activated protein would react causing superoxide product which would then cause NADPH to reduce to NADP+. The researchers would expose the flies to the light for 9 minutes during a 30-minute observation. The flies with the protein were quiescent while the control flies without the protein showed no change in their sleeping patterns. This sleep time was measured by periods of inactivity at least 5 minutes in duration, but a notable effect was that the protein-containing flies showed quiescent periods of over an hour. Also, flies with a removed Hyperkinetic showed resistance to induced sleep.
The implications here are that when the ratio of NADP+ was increased an increase in Shafer activation lead to quiescence. A simplistic rendering of this idea is illustrated here in Figure 1. This induced sleep was found not to occur if the Hyperkinetic group was removed, though. This would support that these groups act like sensors for the ratio of redox reactions and the response in kind begins yet unidentified mechanisms. The same mechanisms that remove the Sandman proteins from the membrane, promote activation of Shaker channels, and result in a stable IA of the dFB neurons.
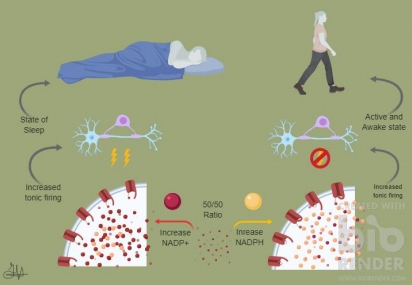
This has implications in making appropriate judgments in sleep disorders. These can occur in anyone and currently impacts around a third of US inhabitants.10 These issues can arise from elevated stress levels as well as physiological conditions like mutated Hyperkinetic subcomponents in Shakers channels. This could improve sleep inducing medication by improving the effectivity of the medication and reducing the side effects. Currently there are various options for sleep inducing drugs. The three main sedative hypnotics are: benzodiazepines, barbiturates, and hypnotics.10 These have issues such as problematic long-term diminishing effects, addictive characteristics, they can lead to fatal overdoses and a host of idiosyncratic effects. There are melatonin targeting medications that have much less severe side effects, but the strength of their effect is less than the other sleep aids.11 The research could indeed develop effective medication to help even insomniacs as well as gain more insight to the mechanisms that control sleeping.
[+] References
Rechtschaffen, A., Gilliland, M. A., Bergmann, B. M., & Winter, J. B. (1983). Physiological correlates of prolonged sleep deprivation in rats. Science, 221(4606), 182-184
Ross, J. J. (1965). Neurological findings after prolonged sleep deprivation. Archives of neurology, 12(4), 399-403
Sherin, J. E., Shiromani, P. J., McCarley, R. W. & Saper, C. B. (1996). Activation of ventrolateral preoptic neurons during sleep. Science 271, 216–219
Hill, V. M. et al. (2018). A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 16, e2005206
Balaban, R. S., Nemoto, S. & Finkel, T. (2005). Mitochondria, oxidants, and aging. Cell 120, 483–495
Orr, W. C. & Sohal, R. S. (1994). Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263, 1128–1130
Donlea, J. M., Thimgan, M. S., Suzuki, Y., Gottschalk, L. & Shaw, P. J. (2011). Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332, 1571–1576
Donlea, J. M., Pimentel, D. & Miesenböck, G. (2014). Neuronal machinery of sleep homeostasis in Drosophila. Neuron 81, 860–872
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13
Side Effects of Sleeping Pills: Common and Potentially Harmful Side Effects. (n.d.). Retrieved 2018, from https://www.webmd.com/sleep-disorders/guide/understanding-the-side-effects-of-sleeping-pills#1
Melatonin: Uses, Side Effects, Interactions, Dosage, and Warning. (2018). Retrieved from https://www.webmd.com/vitamins/ai/ingredientmono-940/melatonin
[+] Other Work By Gavin Harvey
Review on the use of 3D cerebral organoids and the current technical progress in their development
Neuroscience In Review
What do cocktail parties and your reaction to a thunderclap have in common?
Neurophysiology
A new study finds that dopaminergic projections to the medial prefrontal cortex (mPFC) are heavily involved in reactions to aversive or dangerous stimuli.