Disturbances of gamma-band activity and parvalbumin expression in schizophrenia
In schizophrenia, increases in NMDA receptor (NMDAR) inhibition and glutathione (GSH) depletion coexist, but are separate processes. This has led to new insights behind gamma band oscillations and parvalbumin (PV) expression disturbances in schizophrenia and other disorders.
Author: Abby Gligor
Download: [ PDF ]
Neuroanatomy
Schizophrenic pathomechanisms have been thought to involve oxidative stress from decreases of excitatory receptors (NMDA receptors) and an antioxidant, glutathione (GSH), that lead to dysfunctions of specific interneurons that express parvalbumin (PV) and synchronous brain waves called gamma oscillations. However, it was unclear what NMDAR hypofunction and decreased presence of GSH individually contributed to changes in the parvalbumin containing interneursons and gamma oscillations. Until researchers Hasam-Henderson et al. (2018) conducted a study that explored the contributions of NMDARs and GSH each have in the mechanism underlying schizophrenia. They assessed different time points of cultured hippocampal slices during development to see the effects of NMDAR inhibition on oxidative stress and glutathione levels as well as changes in gamma oscillations and PV expression. As a result, inhibition of NMDARs and GSH synthesis both produced oxidative stress. On the contrary, NMDAR inhibition reduced gamma oscillation frequency and the loss of PV was delayed. Depletion of GSH caused immediate decreases in PV and increased power of gamma oscillations. These findings indicate that the different mechanisms coexist, but are separate in schizophrenia.1
Gamma oscillations are synchronous neural activity from information processing and exchange in the brain, which is altered in schizophrenia.2,3 PV, another aspect behind schizophrenia, surrounds GABAergic interneurons and contributes to synchrony by mediating pyramidal cell inhibition.5 Individuals affected by schizophrenia show decreased expression of PV and GABA synthesizing enzyme glutamic acid decarboxylase-67 (GAD67).6,7 Also, it has been reported that genetic deletion of NMDAR disturbs cortical gamma oscillations and decreases PV and GAD67 expressions.8,9 NMDAR hypofunction and oxidative stress have been linked together.10 Oxidative stress contributions in schizophrenia are further supported by decreased levels of the antioxidant, GSH.6,11 Mutations in the synthesis of GSH have been shown to result in decreases of PV expression and altered gamma oscillations.12 It is unclear if network alterations in schizophrenia result directly from NMDAR hypofunction or if they are from the oxidative stress produced by NMDAR hypofunction.
The authors, Hasam-Henderson et al, conducted their study by culturing 750 hippocampal slices from 150 rats for several days. NMDARs and GSH inhibition were pharmacologically done in vitro from day 1 until the assessed time points, which were day 3, 10, or 15. The tissue was assessed for PV using immunohistochemistry and for GSH using a Bradford protein assay. Then, the tissue was imaged using a confocal microscope for visualizing PV. The oxidation of proteins was measured by an oxyblot assay. Electrophysiology was done to observe gamma oscillations.1 All of these assessments were done on day 3, 10, or 15 of being cultured. A summary of the methods is described in Figure 1.
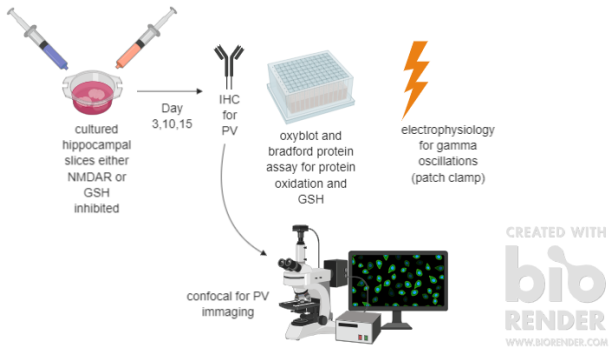
The results of this study indicated several important aspects behind the roles of NMDARs and GSH in oxidation stress, PV expression, and gamma oscillation patterns. First, it was shown that when NMDARs are inhibited, there is an increase of protein oxidation without affecting the levels of GSH. To add on, NMDAR inhibition decreases the frequency of gamma oscillations before affecting PV expression. In another portion of the experiment, oxidative stress alone was shown to disturb PV expression while also increasing the oscillation powers of gamma waves. Another portion showed that a general increase in neuronal activity improved the survival of interneurons even though there was high oxidative stress. Overall, the main findings of this paper was that NMDAR inhibition led to decreased gamma oscillation frequency and a delayed loss of PV, whereas, GSH depletion let to an immediate reduction in PV, but an increase of gamma oscillation power.1
Hasam-Henderson et al provide crucial links to the mechanism of which NMDAR and GSH provide for the oxidative stress, PV expression, and gamma wave oscillations behind the development of schizophrenic brains. Beforehand, it was unclear if NMDAR hypofunction and GSH depletion were part of the same mechanism. Instead, it was found that even though they coexist with each other in schizophrenia, that they are separate processes. This study’s exploration of gamma oscillations can be used for other areas of research such as substance abuse disorder. Information processing and exchange in the brain produce brain oscillations or gamma waves and in substance abuse disorder there is a change in this process. The authors in this paper speculated that the reason PV expression reduced PV, but increased gamma oscillation power could be attributed to the oxidative protective effects of perineuronal nets.13 The nets most likely were degraded in the presence of oxidative stress, which increased neuroplasticity that enhanced gamma oscillations.1 In terms of substance abuse disorder, this paper’s findings open new avenues to researching the mechanism behind addiction using gamma oscillations. Overall, the study conducted provided more information on PV expression, oxidative stress, and gamma wave oscillations as a result from manipulating NMDARs and GSH.
[+] References
Hasam-Henderson, L.A. et al. NMDA-receptor inhibition and oxidative stress during hippocampal maturation differentially alter parvalbumin expression and gamma-band activity. Nature, (2018)
Unhlaas, P.J. & Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100-113, (2013)
Gonzalez-Burgos, G et al. GABA Neuron Alterations, Cortical Circuit Dysfunction and Cognitive Deficits in Schizophrenia. Neural Plansticity, 1-24, (2011)
Traub, R.D. et al. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J. Physio. 493, 471-484 (1996)
Do, K.Q. et al. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 19, 220-230, (2009)
Powell, S.B. et al. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumininterneuron maturation in rodent models of schizophrenia. Neuropharmacology 62, 132201331, (2012)
Belforte, J.E. et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 13, 76-83, (2010)
Korotkova, T. et al. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557-569, (2010)
Cabungcal, J.H. et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 83, 1073-1084, (2014)
Gysin, R. et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence, Proc. Natl. Acad. Sci. USA 104, 16621-16626, (2007)
Steullet, P. et al. Redox disregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 30, 2547-2558, (2010)
Volman, V. et al. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J. Neurosci. 31, 18137-18148, (2011)
Cabungcal, J.H. et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 110, 9130-9135, (2013)
[+] Other Work By Abby Gligor
Brevican: perineuronal net protein captures new insights on interneuron activity
Neurophysiology
Study finds that brevican, a protein found in perineuronal nets and parvalbumin interneurons, is needed for short term memory. This is because brevican regulates the firing properties and type of inputs onto parvalbumin interneurons.