Brevican: perineuronal net protein captures new insights on interneuron activity
Study finds that brevican, a protein found in perineuronal nets and parvalbumin interneurons, is needed for short term memory. This is because brevican regulates the firing properties and type of inputs onto parvalbumin interneurons.
Author: Abby Gligor
Download: [ PDF ]
Neurophysiology
Introduction
In a fascinating paper published in Neuron, Emilia Favuzzi and her team identify the molecular mechanism by which the perineuronal net protein, brevican, influences the cellular and synaptic response of inhibitory neurons to the changing environment.1 The inhibitory neurons they specifically look into are the parvalbumin interneurons located in a region called the hippocampus, which is responsible for learning and memory. The study was conducted because the molecular mechanism behind the adaptability of pavalbumin interneurons to sensory experience is mostly unknown. In addition it also provides more information on how perineuronal nets, a dense net-like structure that surrounds some neurons, influence cellular function. The findings in this study show that brevican is regulated by cellular activity and it controls the excitability of the cell. Even moreso, the study shows that brevican is required for spatial working and short-term memories. Overall, this study provides more information behind the ability of parvalbumin interneurons to adapt to their environment, which is relevant because parvalbumin interneuron dysfunction is linked to various psychiatric disorders.2
Background
First, it is important to understand the complexities behind all the components the study explores and their rationale. For instance, sensory perception, cognition, and behavior are all the result of neural circuits adapting to a continuously changing environment by experiencedependent plasticity. The exact maintenance of these different components is mediated by the balanced interaction of excitation and inhibition.3 In fact, parvalbumin interneurons provide the inhibitory balance in the circuits, which dynamically sustains neural circuit balance.4 Parvalbumin interneurons are able to adapt their intrinsic properties and their outputs during sensory experience.5 Unfortunately, the exact molecular mechanism behind these abilities is largely unknown.
Furthermore, the occurrence of plasticity in the brain relies heavily on the complex interactions of neurons and the extracellular matrix that surrounds them. For parvalbumin interneurons, this matrix is known as perineuronal nets. These nets are largely known to stabilize synapses as well as prevent any new synapses from forming.6 Thus, perineuronal nets block plasticity. Previous studies have shown that degradation of some of the components of perineuronal nets reactivates plasticity and enhances learning.7 However, the exact mechanism behind this is unknown.
Perineuronal nets are made up of chondroitin sulfate proteoglycans.8 One such proteoglycan is brevican. This proteoglycan may be involved in regulating experience-dependent plasticity because it is a component of perineuronal nets, which surround parvalbumin interneurons; it is present in cell membranes and synapse areas; and it is required for long-term potentiation or long-term changes of neuronal synapses.9,10,11 However, brevican’s exact function in parvalbumin interneuron plasticity is unknown.
This study conducted by Favuzzi et al. (2017) bridges the gap of knowledge of the complex interaction between perineuronal nets and parvalbumin interneurons. The knowledge found in this study shows how perineuronal nets and parvalbumin interneurons work together in order to create changes in the neuronal circuitry as a response to sensory experiences.
Results
In order to conduct the study, Favuzzi et al. used various types of mice and techniques to perform various small experiments that comprise the study. They used male mice in each of their various experiments. Some mice genetically expressed brevican while others lacked brevican. The authors sacrificed the animals and stained for brevican, parvalbumin, different sides of the synapse, and other cells using fluorescent tags. Favuzzi et al. also examined the electrical properties of neurons in the absence and presence of brevican. In addition, the authors looked at the protein levels and intensities of various ion channels. Finally, the authors nicely examined the effect of brevican at the single protein level to the behavioral level. They did this by using various mazes such as the T maze and the Y maze. The T maze looks at spatial working memory by using a reward at either end of the T shaped maze and closing off different sides. The animal then has to orientate themselves properly to find the reward. Another maze used was the Y maze. This maze was used to test short term memory by seeing if the animal would like to explore a novel part of the maze after training. Another test was also looking at short term memory by seeing how much time mice spend exploring a new object instead of a familiar object.
Favuzzi et al. found several fascinating results regarding brevican after completing their study. First, they found that brevican is expressed in some glia, but most importantly in parvalbumin interneurons. Brevican is mostly concentrated at the synapse. Another finding was that parvalbumin interneurons have more excitatory inputs onto them when they have brevican compared to cells without brevican. This finding is illustrated in figure 1. In relation to this finding, the authors found that cells that express brevican are less excitable. As noted in another finding, this occurance is because brevican controls the localization and amounts of excitatory receptors and certain ion channels that affect the threshold of cellular activity. The authors also found that brevican decreases with high activity and increases with low activity. Lastly, the authors found that brevican is needed for spatial working and short-term memories.
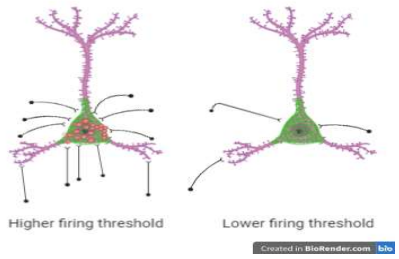
Significance
The study conducted by Favuzzi et al. provides an in-depth evaluation of the perineuronal net protein, brevican, in the adaptable properties of parvalbumin interneurons. In other words, the authors find a molecular mechanism behind the ability of parbalbumin interneurons to change in response to sensory experience using a perineuronal net protein. This finding is important because dysfunctions of parvalbumin interneurons are shown in many psychiatric disorders.2 This work provides a basis of understanding of the role of perineuronal nets in modulating parvalbumin interneuron adaptability.
Personally, I enjoyed the paper and its implications, but I had some skepticism. The study was not clear whether or not parvalbumin cells themselves also expressed brevican or if the brevican is only from the perineuronal nets themselves in the experiments done. Other than that, the paper was a well written and thorough investigation of the role of brevican in parvalbumin interneuron function.
[+] References
Favuzzi, E., Marquez-Smith, A., Deogracias, R., Winterflood, C.M., Sanchez-Aguilera, A., Mantoan, L., Maeso, P., Fernandes, C., Ewers, H., and Rico, B. (2017). Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95, 639-655.
Hu, H., Gan, J., and Jonas, P. (2014). Interneurons, Fast-spiking, parvalbumin’ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263.
Froemke, R.C. (2015). Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci. 38, 195-219.
Xue, M., Atallah, B.V., and Scanziani, M. (2014). Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511, 596-600.
Dehorter, N., Ciceri, G., Bartolini, G., Lim, L., del Pino, I., and Marin, O. (2015). Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science 349, 1216-1220.
Pizzorusso, T., Medini, P., Berardi, N., Chierzi, S., Fawcett, J.W., and Maffei, L. (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248-1251.
Gogolla, N., Caroni, P., Luthi, A., and Herry, C. (2009). Perineuronal nets protect fear memories from erasure. Science 325, 1258-1261.
Deepa, S.S., Carulli, D., Galtrey, C., Rhodes, K., Fukuda, J., Mikami, T., Sugahara, K., and Fawcett, J.W. (2006). Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Bio. Chem. 281, 17789-17800.
Valenzuela, J.C., Heise, C., Franken, G., Singh, J., Schweitzer, B., Seidenbecher, C.I., and Frischknecht, R. (2014). Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130606.
Seidenbecher, C.I., Smalla, K.H., Fischer, N., Gundelfinger, E.D., and Kreutz, M.R. (2002). Brevican isoforms associate with neural membranes. J. Neurochem, 83, 738-746.
Brakebusch, C., Seidenbecher, C.I., Asztely, F., Rauch, U., Matthies, H., Meyer, H., Krug, M., Bockers, T.M., Zhou, X., Kreutz, M.R., et al. (2002). Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation byt show no obvious deficits in learning and memory. Mol. Cell. Bio. 22, 7417-7427.
[+] Other Work By Abby Gligor
Disturbances of gamma-band activity and parvalbumin expression in schizophrenia
Neuroanatomy
In schizophrenia, increases in NMDA receptor (NMDAR) inhibition and glutathione (GSH) depletion coexist, but are separate processes. This has led to new insights behind gamma band oscillations and parvalbumin (PV) expression disturbances in schizophrenia and other disorders.