Schizophrenia with depression: not just a black and white matter
A recent study challenges the classical model for schizophrenia with depression, finding evidence for a unique brain mechanism that is unseen in schizophrenia or depression alone.
Author: Sydney Wolfe
Download: [ PDF ]
Neurophysiology
In an article published by Frontiers in Neuroscience, a team of Chinese researchers investigates the differences between the individual pathways for depression and schizophrenia and the pathways for schizophrenia and depression combined.1 The researchers were able to create experimental groups for depression and schizophrenia using male mice. They found that the group of mice who were administered drugs subsequently for schizophrenia and depression had severe behavioral and neurological impairments. These impairments were especially true when compared to mice who were administered the drugs in the opposite order. If the classical theory for schizophrenia-depression comorbidity was accurate, the order of induction of the disorders should not matter, as the individual pathways for depression and schizophrenia would be additive. Additionally, the administration of antidepressants and antipsychotics to mice with schizophrenia and depression failed to mitigate any adverse psychological effects.1 These findings suggest a possible new pathway for patients diagnosed with schizophrenia, who subsequently experience depression.
A complex disorder
Schizophrenia is a bewildering mental disease, with multiple theories surrounding its causation. Scientists believe schizophrenia stems from physical, genetic, psychological, and environmental factors.2 The current theories surrounding schizophrenia include brain inflammation, NMDAreceptor dysfunction (especially in inhibitory neurons), and redox dysregulation.4.5 Studies have also observed abnormal sulfide production in schizophrenia studies.6,7 The causes for depression are equally elusive, with the primary theories revolving around neuroinflammation, neurogenesis, and the monoamine hypothesis.8,9 Curiously, schizophrenia is often accompanied by other psychiatric ailments, with 50% of schizophrenic patients being diagnosed with depression.3 In the medical and scientific world, schizophrenia and depression are often treated as distinct entities, with distinct pathways.10 However, clinical trials have revealed overlap in the symptoms and effects of comorbid schizophrenia and depression, challenging this classical model.
The experiment
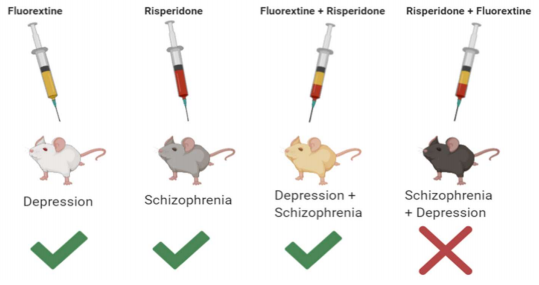
The experimenters used male mice and split them up into five different groups: control, schizophrenia, depression, depression + schizophrenia (subsequently), and schizophrenia + depression (subsequently). The schizophrenia mice were injected with a drug called MK801, also known as dizocilpine, which causes cognitive distortions.1 The researchers used chronic unpredicted mild stress (CUMS) to create a depression model.1 The stressors used included wet bedding, forced swimming, cage tilting, physical restraint, and sleep deprivation.1 The mice performed a variety of behavioral tests, including the forced swimming test and the sucrose preference test. To observe activity in the prefrontal cortex, the researchers measured calcium levels in the live mice. After sacrificing the mice, the researchers observed visual-evoked brain activity and electrophysiological activity in medium spiny neurons (MSNs). These neurons were chosen because behavioral deficits in schizophrenia and depression have been associated with impaired calcium activity in the thalamic nuclei. MSNs, which are heavily localized in the striatum, are thought to initiate or suppress movement, making them a viable target for studying mouse behavior.
Curious findings
The schizophrenia + depression group of mice had the longest immobility time and the lowest preference for sucrose.1 This group demonstrated higher auditory deficits when compared to the other mouse groups.1 Additionally, the schizophrenia + depression group presented the lowest amount of calcium signaling out of all the groups, after antipsychotic and antidepressant administration.1 Paradoxically, visual and behavioral responses were made worse by the drugs. Interestingly, antipsychotic and antidepressant administration mitigated adverse symptoms in the depression + schizophrenia group. This shows possible mechanistic differences between schizophrenia and depression comorbidity and the other treatment groups. Differences in the neural circuit for schizophrenia-depression may explain why classical treatments, such as antipsychotics or antidepressants, are ineffective in treating cognitive/emotional deficits.
Further avenues
This study provides promising evidence for the mechanistic differences between the combined disorder and depression or schizophrenia alone. The two pathways are not simply additive, as previously thought, as drug treatments were ineffectual in the schizophrenia + depression group. Behavioral, auditory, and physical deficits were also exacerbated in this group. Further investigations into the mechanism of schizophrenia + depression are needed to specify which regions of the brain, and what specific neural circuits, might be involved. The use of human trials to image the brains of patients with schizophrenia, who later developed depression, might allow for a more in-depth examination of mouse models by nailing down key brain regions involved in the disorder’s pathophysiology. Gaining insight into these puzzling mental illnesses is crucial to improving drug treatments, and as a result, the quality of life for sufferers.
[+] References
Zhou, C., Kong, D., Zhu, X., Wu, W., Xue, R., Li, G., … Zhuo, C. (2020). Rethinking Schizophrenia and Depression Comorbidity as One Psychiatric Disorder Entity: Evidence From Mouse Model. Frontiers in Neuroscience, 14. doi: 10.3389/fnins.2020.00115
Schizophrenia. (n.d.). Retrieved from https://www.nhs.uk/conditions/schizophrenia/causes/.
Buckley, P. F., Miller, B. J., Lehrer, D. S., & Castle, D. J. (2009). Psychiatric comorbidities and schizophrenia. Schizophrenia bulletin, 35(2), 383–402. https://doi.org/10.1093/schbul/sbn135.
. Steullet, P., Cabungcal, J., Monin, A., Dwir, D., Odonnell, P., Cuenod, M., & Do, K. (2016). Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophrenia Research, 176(1), 41–51. doi: 10.1016/j.schres.2014.06.021.
Nakazawa, K., Zsiros, V., Jiang, Z., Nakao, K., Kolata, S., Zhang, S., & Belforte, J. E. (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology, 62(3), 1574–1583. doi: 10.1016/j.neuropharm.2011.01.022
Simonneau, M. (2019). Looking beyond the usual suspects: sulfide stress in schizophrenia pathophysiology. EMBO Molecular Medicine, 11(12). doi: 10.15252/emmm.201910983.
Ide, M., Ohnishi, T., Toyoshima, M., Balan, S., Maekawa, M., Shimamoto‐Mitsuyama, C., … Yoshikawa, T. (2019). Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Molecular Medicine, 11(12). doi: 10.15252/emmm.201910695.
Jeon, S. W., & Kim, Y.-K. (2017). Inflammation-induced depression: Its pathophysiology and therapeutic implications. Journal of Neuroimmunology, 313, 92–98. doi: 10.1016/j.jneuroim.2017.10.016
Jesulola, E., Micalos, P., & Baguley, I. J. (2018). Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behavioural Brain Research, 341, 79–90. doi: 10.1016/j.bbr.2017.12.025
Anticevic, A., Schleifer, C., & Youngsun, T. C. (2015). Emotional and cognitive dysregulation in schizophrenia and depression: understanding common and distinct behavioral and neural mechanisms. Dialogues in clinical neuroscience, 17(4), 421–434.
[+] Other Work By Sydney Wolfe
Can’t sleep? You might need some stimulation
Neuroanatomy
Scientists discover the therapeutic potential of electrical vagus nerve stimulation in the treatment of primary insomnia.
Circadian rhythm disruption and PACAP-38 in the pathophysiology of cluster headache
Neuroscience In Review