Can’t sleep? You might need some stimulation
Scientists discover the therapeutic potential of electrical vagus nerve stimulation in the treatment of primary insomnia.
Author: Sydney Wolfe
Download: [ PDF ]
Neuroanatomy
The therapeutic effects of transcutaneous auricular vagus nerve stimulation (taVNS) have only recently been explored in the last few years. TaVNS involves the application of electrical stimulation to the external portion of the ear to activate an essential cranial nerve called the vagus nerve.1 In a recent article published in Frontiers in Neuroscience, researchers from the China Academy of Chinese Medical Sciences revealed the immediate effects of taVNS on the brains of patients with primary insomnia (PI).2 They found that patients with primary insomnia showed higher resting-state neural activity than healthy patients, particularly in areas in the cerebral cortex. Interestingly, the application of taVNS to PI patients caused a significant decrease in neuronal activity in these abnormally hyperactive areas. These results provide a promising non-invasive treatment for patients with primary insomnia.
Primary insomnia is becoming an increasingly common issue, with as high as 10% to 15% of the world adult population classifying for a PI diagnosis.3 PI is classified as sleep disturbances that are unrelated to other medical disorders, substance use, or prescription use and must persist for three nights per week for at least three months.3 While most patients are treated using pharmacologic drugs, including benzodiazepines, non-pharmacological methods are being explored as alternatives, such as cognitive-behavioral therapy and taVNS. The therapeutic effects of taVNS have been primarily studied in the context of treatment-resistant depression.7 Clinical studies have shown that taVNS can alleviate many adverse symptoms associated with depression, including anxiety, hopelessness, and sleep disturbance.4, 6, 8 If taVNS can reduce depressive symptoms, one of which includes insomnia, taVNS can be a viable method for treating PI. While the exact mechanism for PI is not entirely understood, many scientists believe that “hyperarousal” is to blame. This theory suggests that patients with insomnia express abnormally high amounts of neurological activity. TaVNS induces a regulatory effect on patients with hyperactive sympathetic activity through stimulation of the vagus nerve.
The experiment
The study observed twenty-two participants with primary insomnia and twenty healthy patients without insomnia. Bilateral electrodes were placed on the auricular concha area for both ears.2 The stimulus frequency was set at 20 Hz, and stimulus intensity varied from patient to patient (4 mA to 6 mA).2 Stimulation was applied for 30 minutes.2 Then, fMRI scans were taken of the PI patients before and directly after taVNS treatment.2 The brains of healthy participants were also imaged once using fMRI.2 The researchers performed two sets of analyses on the brain image: amplitude of low-frequency fluctuations (ALFF) and resting- state functional connectivity (RSFC) analysis.2 These tests aimed to identify any statistically significant differences between brain activity between healthy controls and PI patients.
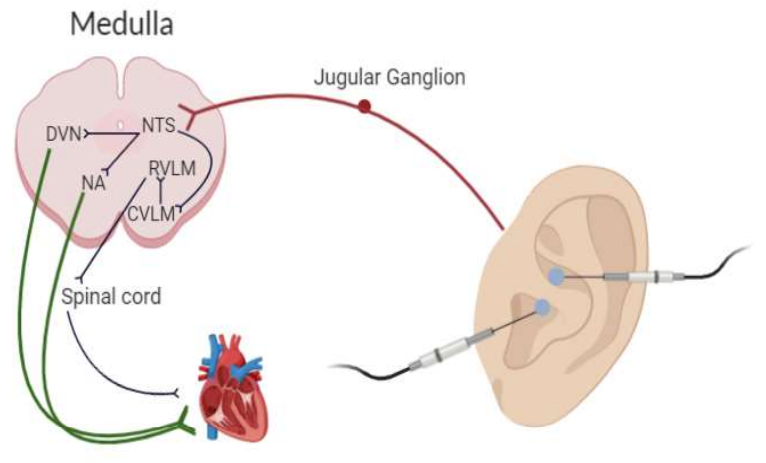
What they found
Significance was measured using a t-test, incorporating voxel quantification from the fMRI scans. Patients with PI had significantly increased ALFF in the right precuneus when compared to the control subjects.2 Following taVNS treatment, ALFF was remarkably decreased in the right precuneus and raised in the left middle occipital gyrus.2 These findings implicated the right precuneus and the right cerebral cortex as regions of interest (ROI). After taVNS, RSFC analysis showed significant decreases in connectivity between the right precuneus and the right superior frontal gyrus, the right middle frontal gyrus, and the right angular gyrus. These results indicate that taVNS may have an unarousing effect on the brain.
Future directions
The exact mechanism by which taVNS operates in patients with primary insomnia is unknown. Studies have uncovered a general mechanism for the pathway of taVNS and how signals project from the ear to the medulla. However, the number of brain areas and pathways activated are far too numerous to attribute one mechanism to insomnia with our current body of knowledge. Further studies will need to explore the functions of the right precuneus and cerebral cortex in sleeplessness and hyperarousal. Additionally, studies will need to identify what medullary structures project to neurons in these ROIs to understand better why taVNS decreases (or increases) frequency fluctuations in these areas. While vagal nerve afferents (to the brain) are known to project to the medulla, vagal nerve efferents (away from the brain) are known to project to the heart, spinal cord, and other vital organs. Studying how these projections might regulate autonomic function may give insight into its therapeutic effects on primary insomnia.
[+] References
Butt, M. F., Albusoda, A., Farmer, A. D., & Aziz, Q. (2020). The anatomical basis for transcutaneous auricular vagus nerve stimulation. Journal of Anatomy, 236(4), 588–611. doi: 10.1111/joa.13122
Zhao, B., Bi, Y., Li, L., Zhang, J., Hong, Y., Zhang, L., … Rong, P. (2020). The Instant Spontaneous Neuronal Activity Modulation of Transcutaneous Auricular Vagus Nerve Stimulation on Patients With Primary Insomnia. Frontiers in Neuroscience, 14. doi: 10.3389/fnins.2020.00205
Momin, R. R., & Ketvertis, K. (2020, February 22). Primary Insomnia. Retrieved April 15, 2020, from https://www.ncbi.nlm.nih.gov/books/NBK554516/
Kong J, Fang J, Park J, Li S and Rong P (2018) Treating Depression with Transcutaneous Auricular Vagus Nerve Stimulation: State of the Art and Future Perspectives. Front. Psychiatry 9:20. doi: 10.3389/fpsyt.2018.00020
Kalmbach, D. A., Cuamatzi-Castelan, A. S., Tonnu, C. V., Tran, K. M., Anderson, J. R., Roth, T., & Drake, C. L. (2018). Hyperarousal and sleep reactivity in insomnia: current insights. Nature and science of sleep, 10, 193–201. https://doi.org/10.2147/NSS.S138823
Kaniusas, E., Kampusch, S., Tittgemeyer, M., Panetsos, F., Gines, R. F., Papa, M., … Šarolić, A. (2019). Current Directions in the Auricular Vagus Nerve Stimulation II – An Engineering Perspective. Frontiers in Neuroscience, 13. doi: 10.3389/fnins.2019.00772
Badran, B. W., Dowdle, L. T., Mithoefer, O. J., LaBate, N. T., Coatsworth, J., Brown, J. C., DeVries, W. H., Austelle, C. W., McTeague, L. M., & George, M. S. (2018). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain stimulation, 11(3), 492–500. https://doi.org/10.1016/j.brs.2017.12.009
Mercante, B., Ginatempo, F., Manca, A., Melis, F., Enrico, P., & Deriu, F. (2018). Anatomo-Physiologic Basis for Auricular Stimulation. Medical acupuncture, 30(3), 141–150. https://doi.org/10.1089/acu.2017.1254
[+] Other Work By Sydney Wolfe
Circadian rhythm disruption and PACAP-38 in the pathophysiology of cluster headache
Neuroscience In Review
Schizophrenia with depression: not just a black and white matter
Neurophysiology
A recent study challenges the classical model for schizophrenia with depression, finding evidence for a unique brain mechanism that is unseen in schizophrenia or depression alone.