Opening the blood-brain barrier
The study examines a novel myelin imaging technique (REMyDI) that was used to discover the amount of myelin in patients with multiple sclerosis and how the quantity was correlated with their physical and cognitive disability ratings.
Author: Alex Tomceac
Download: [ PDF ]
Neuroanatomy
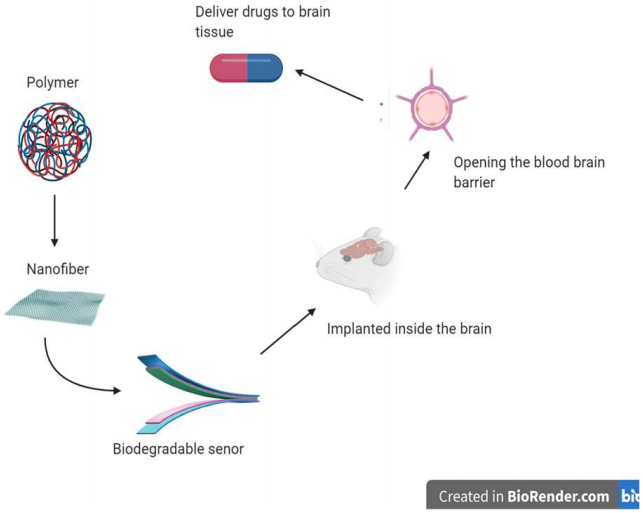
The blood-brain barrier prevents most drugs from entering the brain due to its protective elements and the way it is structured.1 In a recently published article, Nguyen and colleagues developed a technique of using a newly developed biomedical material to utilize for monitoring intra-organ pressure but more importantly, to open the blood-brain barrier.2 The motivation behind this research was to find a more effective device to deliver drugs through the blood-brain barrier, but also a safer way to implement these medical devices without requiring multiple aggressive surgeries that are harmful to the tissues to get to the target location. The primary finding of this research was that the biodegradable device that was created by biomedical engineers is a better way to assist with the delivery of drugs to the brain in localized areas. This is also a new path to avoid invasive surgeries and greatly impact the development of different medical devices in various fields of medicine.2
The human brain has evolved great lengths to keep itself safe and protected from damage. One of the protective elements that the brain has is the blood-brain barrier (BBB). This barrier, as the name, suggests is the physical wall is composed of different cells and cellular components that separate the blood vessels and neuronal tissue. The endothelial cells that line the walls of all blood vessels are structured differently in the vessels of the brain.3 These cells in other parts of the body are fenestrated in the blood vessels, while in the brain, they form tight junctions that only allow small molecules and a few volatile gasses to pass through to the tissue.4 This barrier is also supported by astrocytes and extracellular matrix proteins that are produced by pericytes to further maintain the barrier making it highly selective.5 The BBB is a major obstacle for delivering drugs to the brain for different neurological disorders because these compounds are classified as foreign molecules, therefore, are prevented from passing through to the target tissue.6 Many techniques have been utilized to get passed the BBB, including developing drugs that are lipid-soluble to get delivered to their targets.7 Other new techniques involve using ultrasound are much more effective at opening the BBB by using the vibrations created by a transducer device.8 There are shortcomings with the current ultrasound technology because it is very expensive, and ultrasound waves must be localized on the tissue from multiple sources.9 Another problem with the current transducers devices that are much better at localizing in a specific area is that they require surgery and contain toxic materials such as lead.10 The new device that the bioengineers developed is biodegradable and made of safe materials. It produces localized vibrations to open the BBB for the drugs to pass to the brain tissue successfully.
The piezoelectric transducer is composed of poly L-lactic acid (PLLA) polymer aligned into a specific pattern, and it is activated by an electric circuit that causes the nanofibers to move and vibrate at a high intensity.2 This transducer is then placed through surgical procedure into the brain of a mouse which is connected to erodible wires that are attached to an electrical input to stimulate it. The engineers split the mouse brain into three equal coronal sections (C1, C2, C3). The device was placed in section 2 (C2) and the other sections (C1 and C3) were used as to serve internal control. The control variable in the experiment used a different transducer that was nonpiezoelectric (different material) and had a lower intensity vibration. The effectiveness of the transducer was measured by the concentration of blood protein in neuronal tissue which was coded with fluorescent coloring. A trial consisted of the transducer getting stimulated to vibrate for 30 seconds then a 30-second break.2
Autofluorescence analysis showed higher concentrations of the labeled protein in neuronal tissue in the mice that had the new device implemented. The control produced no similar results showing only low concentrations of the protein. The experiment further ensured that the results are significant and valid by using another drug which also produced the same results. The experimenters analyzed the histology after 2 and 4 weeks after implementation, and results showed that the biodegradable transducer caused a minimal immune response.
This research has many implications, including utilizing these biodegradable materials to engineer medical sensors and transducers for other areas of medicine. The surgical procedure to implement this transducer is much more favorable because the device degrades and does not need to be removed like other transducers. The use of batteries can be eliminated in medical devices in the future if these same materials are used. One of the negative aspects of this is the device requires an external output to produce the desired outcomes. This is still a new field which needs to be further tested to solidify the validity of this experiment.
[+] References
Correale, J., & Villa, A. (2009). Cellular elements of the blood-brain barrier. Neurochemical research, 34(12), 2067.
Curry, E. J., Le, T. T., Das, R., Ke, K., Santorella, E. M., Paul, D., ... & Ko, B. (2020). Biodegradable nanofiberbased piezoelectric transducer. Proceedings of the National Academy of Sciences, 117(1), 214-220.
Wolburg, H., Noell, S., Mack, A., Wolburg-Buchholz, K., & Fallier-Becker, P. (2009). Brain endothelial cells and the glio-vascular complex. Cell and tissue research, 335(1), 75-96.
Abbott, N. J., Rönnbäck, L., & Hansson, E. (2006). Astrocyte–endothelial interactions at the blood–brain barrier. Nature reviews neuroscience, 7(1), 41.
Menezes, M. J., Mcclenahan, F. K., Leiton, C. V., Aranmolate, A., Shan, X., & Colognato, H. (2014). The Extracellular Matrix Protein Laminin 2 Regulates the Maturation and Function of the Blood-Brain Barrier. Journal of Neuroscience, 34(46), 15260-15280. doi:10.1523/jneurosci.3678-13.2014
Pardridge, W. M. (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2(1), 3-14.
Banks, W. A. (2008). Developing drugs that can cross the blood-brain barrier: applications to Alzheimer's disease. BMC neuroscience, 9(3), S2.
N. Vykhodtseva, N. McDannold, K. Hynynen, Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 48, 279–296 (2008).
Angelsen, B. A., & Johansen, T. F. (2003). U.S. Patent No. 6,645,150. Washington, DC: U.S. Patent and Trademark Office
Bernard, Jaffe. "Piezoelectric transducers using lead titanate and lead zirconate." U.S. Patent No. 2,708,244(1955). Neuroscience, 34(46), 15260-15280.
[+] Other Work By Alex Tomceac
Plasticizers Disrupt Neuro Signaling in Adult Brains
Neurophysiology
Readily used plasticizers such as BPA and BPS alter neurotransmission in the brains of goldfish.
The role of neuroinflammation in the progression of Parkinson’s Disease
Neuroscience In Review