Gene mutation heals traumatic brain injury
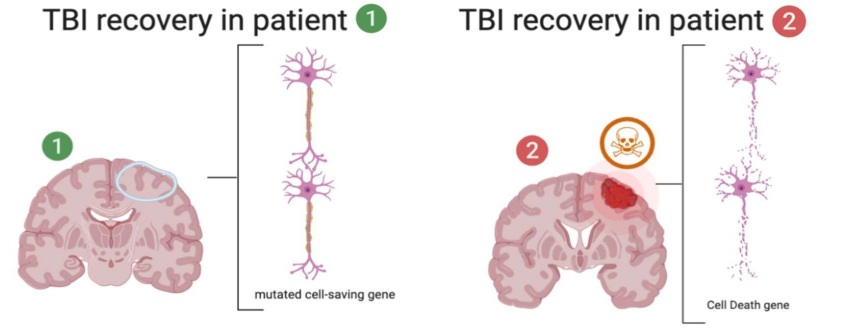
Traumatic brain injuries heal differently for different people. That is because of an apoptotic gene, P53 which is mutated in some people and leads to higher resilience of programmed cell death. Thus, there is increased recovery in severe TBI patients with the mutated P53 gene.
Author: Victor Cosovan
Download: [ PDF ]
Neurophysiology
In a paper recently published in Biological Research for Nursing, Kaleigh Mellett and her team of researchers investigated 429 patients who suffered a severe brain injury and they monitored their DNA from blood to discover if they had any gene mutations. They found that there was a gene with changed language and thus the proteins made from it acted differently. They compared the mutated DNA to the regular human gene for programmed cell death.1 They discovered the mutated gene, it is more successful in stopping programmed cell death leading brain injury, thus giving brain cells the opportunity to recover. Traumatic brain injuries are a leading cause of death with more than 5-million people dying or suffering a disability from it each year.2 Currently, it is not known how to properly treat severe brain injuries and the mechanism of brain healing and recovery needs extensive research to better the outcome of patients who suffer from brain injuries. By looking into the gene for programmed cell death, they found a mutated form of the gene which is more successful at giving damaged cells the opportunity to heal.1
Background
PT53 is the name for the gene that codes programmed cell death.4 When a cell detects a harmful environment(s), faulty DNA, or other cell-deadly factors, it sends out proteins (transcription factors) to wake up DNA and make signals to kill the cell (cell suicide).6 Once the P53 gene is activated it makes proteins which then cause the cell to die and be eaten up as resources by neighboring cells or immune cells. In this study, only subjects who had traumatic brain injuries (TBI) were accessed. TBI is brain damage from force or other harmful environmental causes like drugs or severe temperature. When there is structural damage to the connections between neurons and the cells themselves. That is why when cells detect severe damage, P53 is activated and releases proteins to set the pathway for programmed cell death.8 When a cell is set for programmed cell death, excess proteins and enzymes will flow through the brain circulatory pathway (cerebral spinal fluid) and drain into the blood to be metabolized and discharged as waste.10 Researchers are able to then collect blood samples from severe TBI patients and locate and analyze P53 genes and proteins. The gene mutation is called Arg72Pro TP53 because on the long amino acid strand (DNA alphabet and language), there is a typo on the 72nd amino acid where a proline got replaced by an arginine molecule. By finding two different types of P53, scientists are able to compare the outcomes in patients who have the mutated version and how well they heal because of reduced cell death.1 Neurons take a long time to heal and build new connections and pathways when one is damaged. They function as a series of bridges that move and form other bridges too. When there is structural change, there is mass inflammation which then needs to be controlled because the brain is encapsulated in a tight compartment, the skull.
Methods
This study took blood or cerebral spinal fluid samples from 429 participants suffering from a severe traumatic brain injury (TBI). They selected participants off of a particular criterion. The participants were adults ages 16-80, taken only with a Glasgow Coma Scale (GCS) score greater than or equal to 8.3 The participants couldn’t have any brain injury related to any form of food or drug. During 3, 6, 12, and 24 months, a team of neurophysiological technicians performed a class of neurological exams. They tested using mortality and scores on the Glasgow Outcome Scale (GOS), Neurobehavioral Rating Scale (NRS), and Disability Rating Scale (DRS). They monitored and tested; alertness, attention, fatigability, orientation, memory, motor behavior, expressive/reception language, mood disturbances, disinhibitory behavior or agitation, and capacity for self-insight.1
Results
In this study, they found two forms of the P53 gene, a wild type, and a mutated type, Arg72Pro TP53.1 They found that the mutated gene patients had a significantly lower mortality rate than those with the wild type activated P53 gene. They found that in their subjects, after 24-months post TBI event, there was a significant difference in NRS and DRS score outcomes in the two patients groups using X 2 tests. They also found a significant difference in outcome of patients who started off with a GCS 3-4 vs those who started at a better 5-8 score at 24 months postTBI. Age was also significant for NRS and GOS at 24 and 3 months post-TBI. The study found that patients with the proline (wild type) gene and had significantly worse outcomes than those with the arginine mutation using GOS scores to compare and analyze statistically.7 The mutated TP53 gene is more efficient at inducing apotosis.4
Significance
There were many important findings in this article.1 They study how the mutated gene compares to the wild type gene in multi-variable analyses. The first finding was the significant decrease in mortality rate across mutated patients and wild type patients. This is an important finding because it shows that treating apoptotic functions in patients with severe TBIs can lead patients to have an increased survival rate.11 This opens the door to medicine to treat genotype-based precision medicine in patients with varying neurodegenerative illnesses. Patients with the wild type gene were 2.7 times more likely to have a poor outcome compared to patients10 with the induced apoptotic function.1 Meaning if we can use genotypic medicine or pharmacological inhibition of apoptosis in cells, there can be a 2.7 times greater chance of survival in severe TBI patients. This is a great study with a large sample size and many well-though-out positive and negative control tests. The study holds great validity and took extra time to compare all variables and statistically evaluate all of them. They also plugged from previous studies and filled holes by using multi-variable analysis to assess the differences in sex and age in patients with severe TBIs. This study now opened the door to new studies searching for ways to efficiently induce apoptosis to increase neurophysiological outcomes. I only wish the study specified how many patients they collected blood samples from and how many patients they collected CSF from. Then do control analysis on the two sample types. I consider this a well written valid study.
[+] References
Mellett, K., Ren, D., Alexander, S., Osier, N., Beers, S. R., Okonkwo, D.O., ... & Conley, Y. P. (2020). Genetic Variation in the TP53 Gene and Patient Outcomes Following Severe Traumatic Brain Injury. Biological Research for Nursing, 1099800420912335.
Centers for Disease Control and Prevention. (2019). TBI: Get the facts. https://www.cdc.gov/traumaticbraininjury/get_the_facts.html.
Christensen, B. (2014). Glasgow Outcome Scale. https://emedicine.medscape.com/article/2172503-overview.
Dumont, P., Leu, J. I., Della Pietra, A.C., 3rd, George, D. L., & Murphy, M. (2003). The codon 72 polymorphic variants of P53 have markedly different apoptotic potential. Nature Genetics, 33(3), 357–365. https://doi.org/10.1038/ng1093.
Faul, M., Xu, L., Wald, M. M., & Coronado, V.G. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control.
Genetics Home Reference. (2020). TP53 gene. https://ghr.nlm.nih.gov/gene/TP53#location
Vanier, M., Mazaux, J. M., Lambert, J., Dassa, C., & Levin, H. S. (2000). Assessment of neuropsychologic impairments after head injury: Interrater reliability and factorial and criterion validity of the Neurobehavioral Rating Scale-Revised. Archives of Physical Medicine and Rehabilitation, 81(6), 796–806. https://doi.org/10.1016/s0003- 9993(00)90114-x
He, J., Wang, F., Zhu, J., Zhang, Z., Zou, Y., Zhang, R., Yang, T., & Xia, H. (2017). The TP53 gene rs1042522 C>G polymorphism and neuroblastoma risk in Chinese children. Aging (Albany NY), 9(3), 852–859. https://doi.org/10.18632/aging.101196.
Kaya, S. S., Mahmood, A., Li, Y., Yavuz, E., Goksel, M., & Chopp, M. (1999). Apoptosis and expression of p53 response proteins and cyclin d1 after cortical impact in rat brain. Brain Research, 818(1), 23–33. https://doi.org/10.1016/s0006-8993(98)01204-9.
Morrison, R. S., Wenzel, H. J., Kinoshita, Y., Robbins, C. A., Done- hower, L. A., & Schwartzkroin, P. A. (1996). Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. Journal of Neuroscience, 16(4), 1337–1345.
Yang, L. Y., Greig, N. H., Huang, Y. N., Hsieh, T. H., Tweedie, D., Yu, Q. S., Hoffer, B. J., Luo, Y., Kao, Y. C., & Wang, J. Y. (2016). Post-traumatic administration of the p53 inactivator pifithrin-alpha oxygen analogue reduces hippocampal neuronal loss and improves cognitive deficits after experimental traumatic brain injury. Neurobiology of Disease, 96, 216–226. https://doi.org/10.1016/j. nbd.2016.08.012.
[+] Other Work By Victor Cosovan
Functional prions are in the central nervous system; how they influence memory!
Neuroanatomy
Prions are an incredible class of proteins which offer many beneficiary functions to the central nervous system, such as cytoplasmic polyadenylation element-binding protein (CPEB), its role in long-term memory and synaptic plasticity. Prions also serve as a harmful misfolded protein within the nervous system, this caused all prions to classically be identified as the forefront for neurodegenerative illnesses. TIA-1 is also a crucial functioning prion and it’s known for its role during stress in the brain and programed cell death. It is now known to be a key member of normal cell biology.
Structural and functional prion malfunction as a catalyst for Alzheimer’s disease
Neuroscience In Review