Did I Forget to Breathe? A Literature Review on how Breathing Affects our Memories
Author: Iris Gutierrez
Neuroscience In Review
Introduction
Breathing is an essential part of our lives and suffering from a breathing disorder can have detrimental effects. In 2017, about 544.9 million people suffered from a chronic breathing disorder such as Chronic Obstructive Pulmonary Disease, asthma, and other breathing disorders.1,2 Other breathing abnormalities include breathing through the mouth versus the nose. The rates for mouth breathing are about 50-56% for children and rates sharply decline for adults.1,2
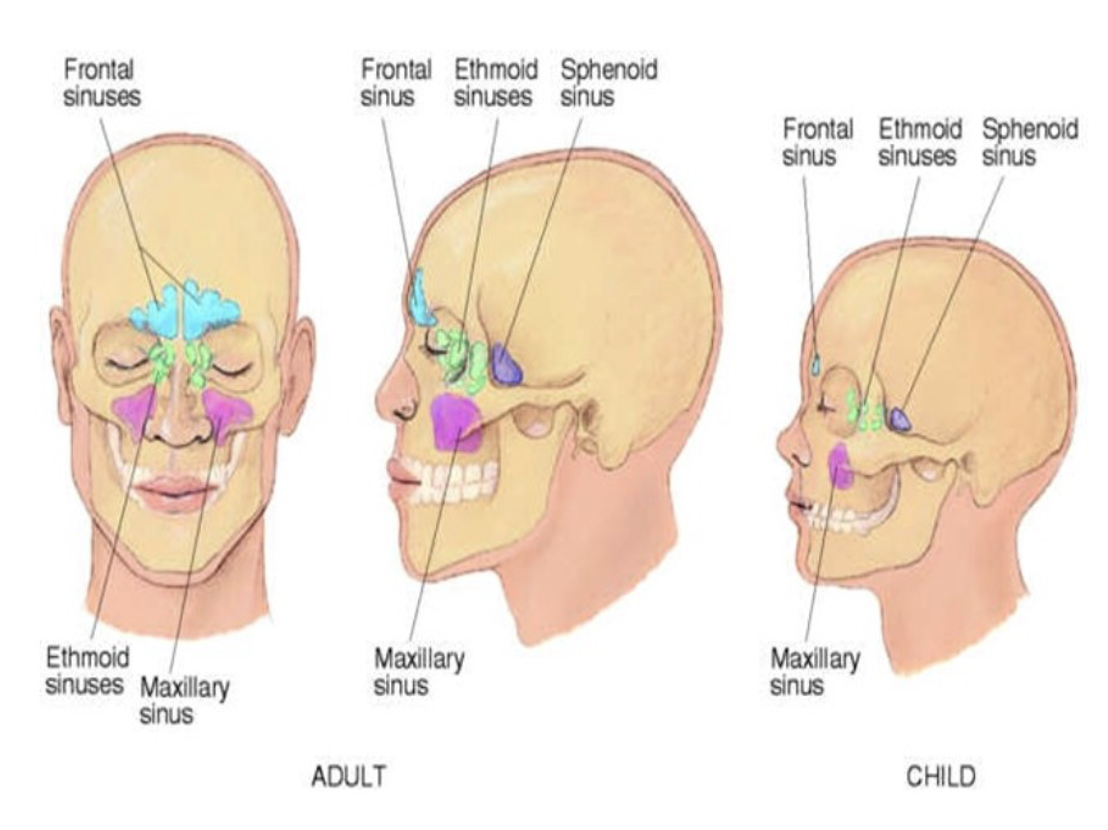
This decline in mouth breathing rates between children and adults is mainly due to anatomical differences as well as some environmental differences. The anatomical differences include the lack of development in children’s sinuses. There are 4 sinuses present that develop over time. Two of the sinuses (the ethmoid and maxillary) are present at birth and they continue to grow with age, while the frontal sinus does not develop until age 7, and the sphenoid sinus does not develop until adolescent age.3 The lack of sinus development along with environmental factors (such as allergies or foreign objects stuck in the nasal passages) are what make children more susceptible to sinusitis or common cold-like symptoms that may lead the child to rely on breathing through their mouth.4
For adults, the sinuses are well developed, and some environmental factors (such as shoving objects in the nose) are decreased.3 This decreases the dependence on mouth breathing (aside from the occasional nasal congestion). However, long-term reliance on mouth breathing is being investigated and some studies found that mouth breathing (vs. nose breathing) reduces memory functioning.5,6,7,8,9
Some studies have looked at the implications of breathing modality (mouth vs. nose) on memory functions and found that nose breathing facilitates memory functioning because of the nose’s anatomy and the way it channels air into the body, as well as the systematic communication in the brain by these rhythmic breaths (the PreBötzinger Complex and its connections with the locus coeruleus).5,6,7,8,9,10 This review evaluates the impacts of breathing modality on memory function.
The PreBötzinger Complex & Its Fling with the Locus Coeruleus
The PreBötzinger Complex (PreBöC) is regarded as the “respiratory pacemaker” because of its ability to generate rhythmic signals during respiration (specifically during inspiration).10,11These signals are transmitted in both ascending and descending pathways, where ascending pathways signal other areas in the brain, and descending pathways stimulate motor neurons of respiratory muscles.10,11
In ascending pathways, the other brain areas that receive rhythmic signals include the locus coeruleus (LC); the LC is then responsible for attention, arousal, and sleep-wake transitions. The LC projects into the hippocampus, along with other brain regions.10,11 This is the general pathway of how breathing creates rhythmic signals that are transmitted elsewhere, but is this pathway affected with the change in breathing modality? Turns out, this is the case.
Breathing modality changes the rhythmic signals being generated in the PreBöC and therefore the rhythmic signals transmitted to the hippocampus. Just like the outer ear, the nose is anatomically capable of channeling air for the proper rhythmic signals. A study evaluating breathing modality found that respiratory signals in the hippocampus were significantly decreased when breathing was diverted from the nose to the mouth.5 The study also evaluates visual and episodic memory deficits in the encoding or retrieving phases.5 The participants that were oral breathers experienced significant memory deficits in the encoding and retrieving phases due to decreased rhythmic signals.5 Other studies have looked at the effects of damage to the PreBötC on rhythmic signals and found that respiration was inhibited and resulted in memory and cognitive deficits.5,14 One study looking at laser ablation of the PreBötC in mice found that mice with significant damage were unable to draw a single breath.12,13 Due to experiencing deficits in both PreBötC ascending (memory deficits) and descending (lack of muscle movement) pathways, this further supports the idea of the PreBötC as the pacemaker of the respiratory system.
Other studies have also found that breathing modality made a significant difference when it came to recognition of smells.15 Participants that engaged in oral breathing had significantly reduced recognition of olfactory memory than participants that engaged in nasal breathing.15
While the rhythmic signals affected more brain areas than just the hippocampus, the hippocampus experienced the most repercussions detailed in intracranial EEG imaging that resulted from oral breathing.5 Perhaps these findings indicate that hippocampal activity is dependent on these respiratory rhythmic signals.
Hippocampal Oscillations & Their Role in Memory Consolidation
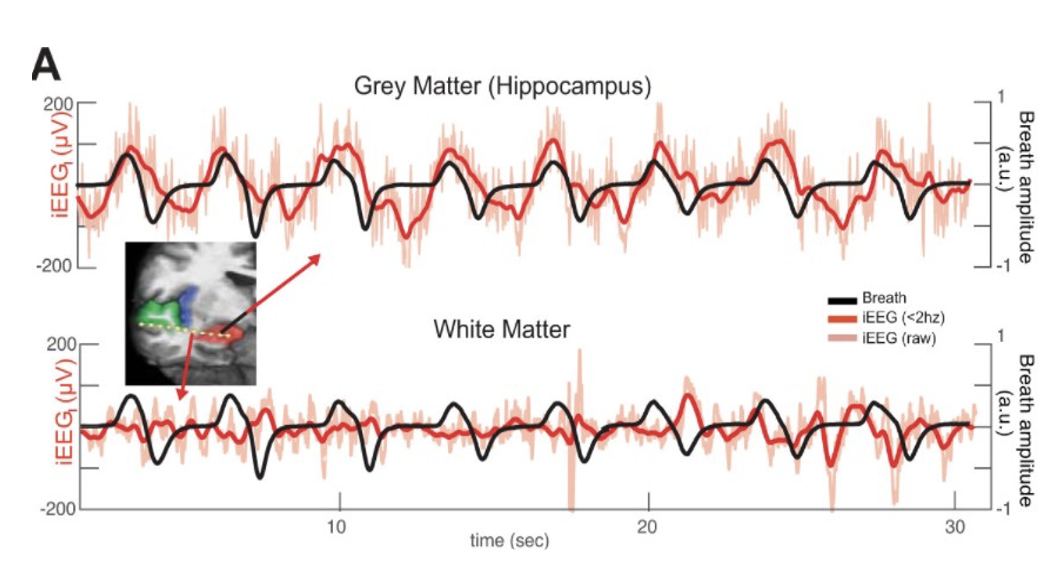
Respiration can play a vital role in hippocampal activity/oscillations and therefore can alter memory functioning and consolidation. A study published in the Journal of Neurophysiology depicts a relationship between breathing modality and memory. The researchers evaluated the hippocampal oscillations and breathing rate of nose breathers and found that hippocampal oscillations appear synched with breathing rate when compared with other brain areas.11,14 Oscillations recorded from the white matter did not experience the same synchronized activity.11,14 The researchers also evaluated the predominant frequencies within each recording and the coherence level and found that breathing rate and hippocampal activity share a peak and resulted in a higher coherence level.11,14 Typically, higher coherence indicates higher synchronicity between breathing rate and hippocampal oscillations.14,16 This is additionally evaluated in another study and high coherence was not apparent in individuals that breathe orally.16 In these individuals, there is low coherence.16 Another study found that higher coherence levels are tied to increases in sharp wave ripples (SWR) in the hippocampus and that hippocampal damage can lead to retrograde and anterograde amnesia.18 This study looked at mice and found that these SWRs are higher during slow-wave sleep after mice were exposed to new environments when compared to familiar environments.18 Interestingly, other studies that disrupt the SWRs saw slower learning in hippocampus-dependent spatial memory tasks.19,20
Overall, the synchronicity from hippocampal oscillations increases sharp wave ripples (SWR) that are essential for memory consolidation.
What This Means for Sleep & Memory Consolidation + Future Directions
If oral breathing reduces memory functioning in our wake lives, what does that mean when we are asleep? Disorders such as sleep apnea can result in memory deficits due to lack of respiratory oscillations.17,19 In fact, a study looking at the silencing of preBötC neurons in rats led to sleep apnea and led to researchers providing mechanical ventilation.17,19 Similarly, patients with Obstructive Sleep Apnea (OSA) experienced significantly lower levels of episodic, and verbal memory functioning.17 Interestingly, medications that help boost the PreBötC signals when damage is present have also been found to help with sleep apnea.17 Sleep is very important for memory consolidation, but many things are still unknown.
After all of this research, why isn’t oral breathing considered a respiratory disorder alongside COPD and asthma? Perhaps the reason is because these other disorders take priority over oral breathing and leads to oral breathing not being addressed or treated. Perhaps treatments should target the PreBötC and its rhythms to combat both respiratory disorders and memory deficits. The future directions include a longitudinal study on children and evaluate rhythms generated from the PreBötC as they age and determine whether oral breathing children present higher rates of sleep apnea, OSA, and/or memory deficits while experiencing more non-synchronous/ rhythmic activity in the hippocampus.
[+] References
GBD Chronic Respiratory Disease Collaborators (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. Respiratory Medicine, 8(6), 585–596. https://doi.org/10.1016/S2213-2600(20)30105-3
Abreu, Rocha, Lamounier, & Guerra (2008). Prevalence of mouth breathing among children. Journal de pediatria, 84(5), 467–470. https://doi.org/10.2223/JPED.1806
Boston Children's Hospital. (n.d.). Sinusitis. Boston Children's Hospital. Retrieved from https://www.childrenshospital.org/conditions/sinusitis
Pacheco, Casagrande, Teixeira, Finck, & de Araújo (2015). Guidelines proposal for clinical recognition of Mouth Breathing Children. Dental press journal of orthodontics. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4593528/
Zelano, Jiang, Zhou, Arora, Schuele, Rosenow, & Gottfried (2016). Nasal respiration entrains human limbic oscillations and modulates cognitive function. Journal of Neuroscience. Retrieved from https://www.jneurosci.org/content/36/49/12448?ijkey=9755bfd8bc7250163a50953b353e5997bf1ac17e&keytype2=tf_ipsecsha
Heck, D. H., Kozma, R., & Kay, L. M. (2019). The rhythm of memory: how breathing shapes memory function. Journal of Neurophysiology, 122(2), 563–571. https://doi.org/10.1152/jn.00200.2019
Lee, Park, Lee, Kim, & Kang (2020). Eeg signals during mouth breathing in a working memory task. International Journal of Neuroscience, 130(5), 425-434. doi:http://dx.doi.org/10.1080/00207454.2019.1667787
Kuroishi, Garcia, Valera, Anselmo-Lima & Fukuda (2015). Deficits in working memory, reading comprehension and arithmetic skills in children with mouth breathing syndrome: analytical cross-sectional study. Revista paulista de medicina, 133(2), 78–83. https://doi.org/10.1590/1516-3180.2013.7630011
McKeown, & Macaluso (2020). Mouth breathing: Physical, mental and emotional consequences. Oral Health Group. Retrieved from https://www.oralhealthgroup.com/features/mouth-breathing-physical-mental-emotional-consequences/
Karalis & Sirota (2018). Breathing coordinates limbic network dynamics underlying memory consolidation. bioRxiv. Advance online publication. Retrieved from doi:10.1101/392530. doi:10.1101/392530
Yackle, Schwarz, Kam, Sorokin, Huguenard, Feldman, Luo & Krasnow (2017). Breathing control center neurons that promote arousal in mice. Science. Retrieved from https://www.science.org/doi/10.1126/science.aai7984
Wang, Hayes, Revill, Song, Kottick, Vann, LaMar, Picardo, Akins, Funk & Negro (2014). Laser ablation of DBX1 neurons in the pre-Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. eLife. Retrieved from https://elifesciences.org/articles/03427
Vann, Nikolas & Pham, Francis & Hayes, John & Kottick, Andrew & Negro, Christopher (2016). Transient Suppression of Dbx1 PreBötzinger Interneurons Disrupts Breathing in Adult Mice. PloS one. 11. e0162418. 10.1371/journal.pone.0162418.
Herrero, The Feinstein Institute for Medical Research, Khuvis, Yeagle, Cerf, Interdepartmental Neuroscience Program and Kellogg School of Management, Mehta, Lindsey (2018). Breathing above the brain stem: Volitional control and attentional modulation in humans. Journal of Neurophysiology. Retrieved from https://journals.physiology.org/doi/full/10.1152/jn.00551.2017
Arshamian, Iravani, Majid, & Lundström, (2018). Respiration modulates olfactory memory consolidation in humans. Journal of Neuroscience. Retrieved from https://www.jneurosci.org/content/38/48/10286?ijkey=ddc62f59be562eeee88d5b89df82ba904daf063a&keytype2=tf_ipsecsha
Bowyer (2016). Coherence a measure of the brain networks: Past and present - neuropsychiatric electrophysiology. BioMed Central. Retrieved from https://npepjournal.biomedcentral.com/articles/10.1186/s40810-015-0015-7#:~:text=Coherence%20is%20a%20mathematical%20technique,patterns%20of%20oscillating%20brain%20activity.
Wallace, Bucks (2013). Memory and obstructive sleep apnea: A meta-analysis. Sleep. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543053/
Joo & Frank (2018). The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nature News. Retrieved from https://www.nature.com/articles/s41583-018-0077-1
Ego-Stengel & Wilson (2010). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus, 20(1), 1–10. https://doi.org/10.1002/hipo.20707
Girardeau, Benchenane, Wiener, Buzsáki, & Zugaro (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nature Neuroscience, 12(10), 1222–1223. https://doi.org/10.1038/nn.2384
[+] Other Work By Iris Gutierrez
How Gaps Between Cells Can Help Fight Cancer
Neuroanatomy
A new study shows that gaps between cells can be the next tool to fight cancer development due to electricity properties.
Our Brains Stay Young by using Cruise Control
Neurophysiology
Study shows that calcium channels can make our brain more excitable by turning on cruise control and accelerating like a car on the freeway