Pf-STN Brain Pathway: a Potential New Target for Parkinson’s Treatment
Author: Alejandra Silva Hernandez
Neuroanatomy
A defining characteristic of Parkinson’s disease (PD) is the loss of control of voluntary motor movement and poor quality of life. The loss of control arises from a loss of dopamine, a critical neurotransmitter used by the brain to regulate voluntary motor movement like walking, eating, and other coordinated movements. Currently, there is no cure for Parkinson’s disease, but its symptoms can be managed. According to Mayo Clinic, exercise, medication and surgery are treatment options that improve symptoms. The treatment plan will vary from patient to patient, as each will have specific symptoms, and a tailored treatment regime should be chosen to manage their symptoms (Mayo Foundation for Medical Education and Research, 2022)
In general, PD medications work by increasing the concentration of dopamine in the brain or replacing the loss of dopamine with a synthetic substitute. According to Parkinson’s Foundation, medications tend to restore movement fluidity, speed, and coordination. A commonly used PD medication is Levodopa, its side effects include low blood pressure, nausea, confusion, dyskinesia or involuntary movements. Parkinson’s disease: Medication for Parkinson's disease (2015) reports that current treatment for Parkinson’s disease in patients are not entirely effective and/or lose their efficiency in treating symptoms with time so another option is surgery.
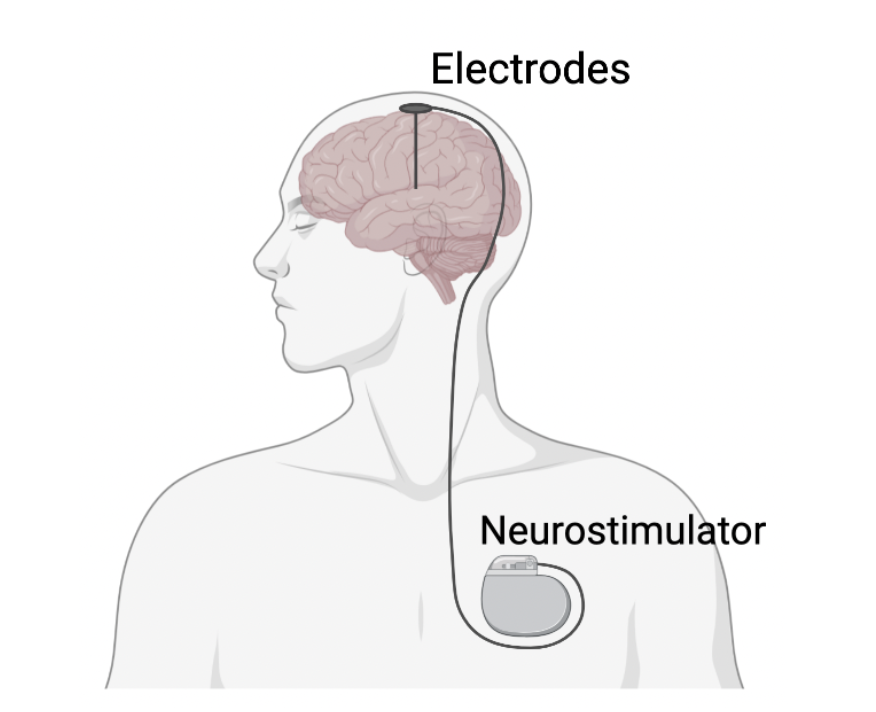
According to the Parkinson’s Foundation, Deep Brain Stimulation (DBS) is an FDA approved surgical procedure used to treat patients with tremors due to poorly controlled Parkinson’s disease. Patients who are eligible for DBS treatment can continue their anti-Parkinson's medication, such as Levodopa. If DBS treatment is successful, then physicians can lower their patients’ medication dosage and reduce their exposure to potential side effects, like dyskinesia. Eligible patients are typically those “who have had PD for at least four (4) years and have motor symptoms not adequately controlled with medication”. A small opening is surgically created on the skull to implant the DBS device. Electrodes are placed in the target brain region and a pacemaker-like battery, neurostimulator, is implanted into the collarbone or abdomen, which patients can turn on and off. Currently DBS is approved for surgical implantation into the subthalamic nucleus (STN) and the globus pallidus International (GPi) (Parkinson's Foundation, n.d.).
In Thalamic projections to the subthalamic nucleus contribute to movement initiation and rescue of parkinsonian symptoms, researchers report a new potential target for treating Parkinson’s disease: the Pf-STN brain pathway. The parafascicular nucleus (Pf) and subthalamic nucleus (STN) are located in the center of the brain and are associated with voluntary movement (Lanciego, Luquin, & Obeso, 2012). Researchers in this study ran experiments to ascertain the direction of connectivity between brain regions. They differentiated which neurons were activated, and to what degree, to determine brain region of interest. From these results, researchers chose to focus on the projections from the parafascicular nucleus to the subthalamic region.
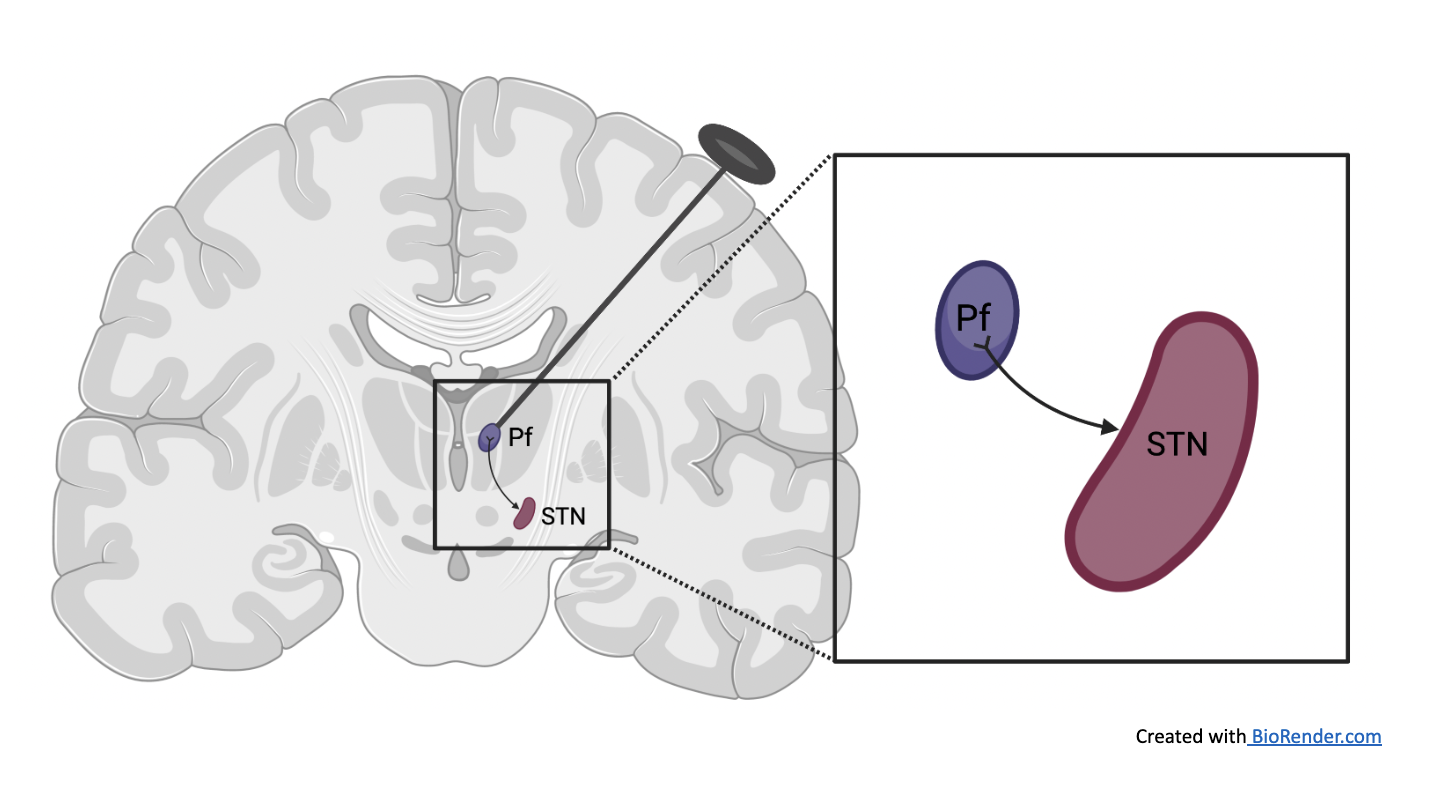
Methods used to stimulate the Pf-STN pathway in this study required craniotomies or brain surgeries in mice which were conducted under anesthesia. Optic fibers were implanted and secured, and mice were allowed to recover for one week before experiments started. Stimulation was triggered randomly in 12 to 15 second intervals, and movement was tracked with infrared cameras to a millimeter scale. MATLAB program was used to analyze the data. The degree of mobility was analyzed and characterized into predetermined behavioral categories, before and after stimulation.
Interestingly, stimulation of the Pf-STN pathway was sufficient to restore movement and orientation in PD mice. PD mice were seen to travel significantly longer distances when Pf-STN region was stimulated than when it was not. Deep brain stimulation that specifically targets the parafascicular-subthalamic nucleus pathway was shown to help control movement in mice with parkinsonian like symptoms (PD mice).
This is an exciting new prospect for Parkinson’s disease treatment. Patients' quality of life is severely reduced with PD and having another Deep Brain Stimulation area to target can only help additional patients. However, additional studies would need to be conducted. This study used a mouse model for Parkinson’s disease which might not sufficiently represent human disease and treatment response. Perhaps, other animal models could be tested before human clinical trials. By exploring further research into stimulating this new brain circuit, patients with Parkinson’s disease might have another treatment target for Deep Brain Stimulation to improve their quality of life.
[+] References
Deep Brain Stimulation (DBS). Parkinson's Foundation. (n.d.). Retrieved May 3, 2022, from https://www.parkinson.org/Understanding-Parkinsons/Treatment/Surgical-Treatment-Options/Deep-Brain-Stimulation
Hanini‐Daoud, M., Jaouen, F., Salin, P., Kerkerian‐Le Goff, L., & Maurice, N. (2022). Processing of information from the parafascicular nucleus of the thalamus through the basal ganglia. Journal of Neuroscience Research, 100(6), 1370–1385. https://doi.org/10.1002/jnr.25046
InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Parkinson’s disease: Medication for Parkinson's disease. 2015 Apr 8 [Updated 2019 Jul 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK293715/
Lanciego, J. L., Luquin, N., & Obeso, J. A. (2012). Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine, 2(12), a009621. https://doi.org/10.1101/cshperspect.a009621
Levodopa. Parkinson's Foundation. (n.d.). Retrieved May 3, 2022, from https://www.parkinson.org/Understanding-Parkinsons/Treatment/Prescription-Medications/Levodopa
Mayo Foundation for Medical Education and Research. (2022, March 24). Parkinson's disease. Mayo Clinic. Retrieved May 3, 2022, from https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/diagnosis-treatment/drc-20376062
Watson, G. D., Hughes, R. N., Petter, E. A., Fallon, I. P., Kim, N., Severino, F. P., & Yin, H. H. (2021). Thalamic projections to the subthalamic nucleus contribute to movement initiation and rescue of parkinsonian symptoms. Science Advances, 7(6). https://doi.org/10.1126/sciadv.abe9192