Introduction of Exosomes Proves to Prompt Myelin Regeneration
Regeneration of the peripheral nervous system’s myelin sheaths proves to be a possibility in the near future, promoting the start of a potential cure for neurodegenerative diseases.
Author: Noël Smith
Neuroanatomy
Diseases that progress through the occurrence of demyelination often have a grim prognosis. Peripheral nerve injuries that spur demyelination can be fatal. A few well known associated diseases are Parkinson’s Disease, Multiple Sclerosis (Swanson, 2020), Lou Gehrig’s Disease, and Guillain-Barre Syndrome (Gu et al., 2014). The strides modern medicine has made to alleviate symptoms has been notable, but repairing the structural damage, up until recently was thought to be impossible. If myelin sheaths were able to be repaired and the degeneration of the sheaths ceased, a cure for the aforementioned diseases could be getting closer. A recent study published in the International Journal of Biochemistry and Cell Biology reported that not only were they able to observe a decrease in demyelination through the introduction of a specific type of tissue, but were also able to establish the mechanism by which this advancement occurs (Yin et al., 2021). This could be the beginning of drastically changing the course of these highly progressive diseases.
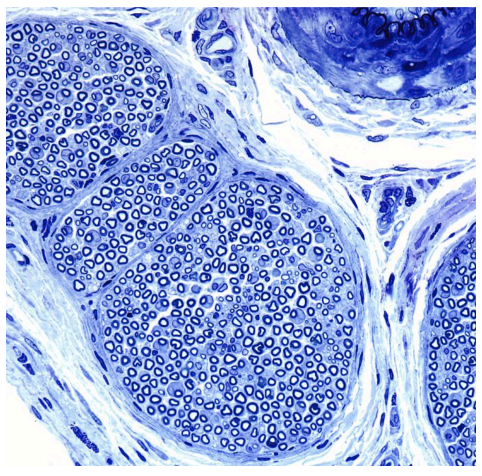
Nerves’ axons are encased with Schwann cells, which comprise a significant portion of the peripheral nervous system (Madduri, 2010). They are also referred to as myelin sheaths. They facilitate conduction and insulate the axonal fibers from extracellular space
(Gomez-Sanchez et al., 2015), with exception to the aperture between each Schwann cell. This gap provides access for excitation of the axonal membrane. The low capacitance of the myelin sheaths gives way to active excitation and impulse propagation at a pace much faster than that of unmyelinated axons (Morrell & Quarles, 1999). When these sheaths are damaged, removed, or displaced, the
propagated signal can be lost due to insufficient innervation. As is the case in many peripheral nerve injuries, when there is damage, the activation of Wallerian degeneration will begin a cycle of myelin disintegration resulting in the disruption of axonal connections (Namikawa et al., 2006). Schwann cells signal macrophages to clean up the myelin and axonal debris through phagocytosis (Gaudet et al. 2011).
This autophagy occurs in both normal development and in neurodegenerative diseases (Wong et al., 2017). Another study from the
Journal of Cell Biology presented findings that myelinophagy, the clearing of myelin by autophages, is reprogrammed after an injury and
therefor is defective, accelerating myelin clearance (Koch et al., 2010), (Gomez-Sanchez et al., 2015). This results in a speedy progression of neurodegenerative diseases (Ding et al., 2007). Past studies have shown that the introduction of adipose-derived stem cells promote regeneration after peripheral nerve injury (Jessen et al., 2015). The study from International Journal of Biochemistry and Cell Biology takes a closer look at the effects and mechanisms of exosomes produced by adipose-derived stem cells on the autophagy of Schwann cells in peripheral nerve injury and their effects on the regeneration of the myelin sheaths (Yin et al., 2021). Their results show that adipose- derived stem cell exosomes do in fact promote regeneration of the myelin sheath by reducing autophagy.
A number of male adult rats were purchased and sorted into two groups. In the Sciatic nerve injury group, 36 rats were anesthetized and
while under sedation had their sciatic nerve crushed in a consistent location, patter, and and with consistent pressure. The injury was biomarked and a myoelectric examination was performed after half an hour. It showed the motor conduction velocity of the
sciatic nerve had fallen below a typical level, and thus, the injury was determined to be successful. The sham group consisted of 15 rats. They were also anesthetized, and an incision was made to expose the sciatic nerve, but no injury was inflicted. From a third group of rats,
adipose-derived stem cells were isolated, purified and characterized by immunofluorescence staining and flow cytometry. The exosomes were then extracted and analyzed by transmission electron microscopy. Wallerian degeneration became visible about 24 hours after the injuries were inflicted. Schwann cells were isolated from the injury site of the first group of rats. Eventually adipose-derived stem cells were transfected with an miRNA-26b inhibitor that acts as a negative control. The proteins extracted from the sciatic nerve were separated, fixed, and mounted two be viewed with transmission electron microscopy while quantitative real-time reverse transcriptase PCR was used to synthesize the RNA found in the exosomes. Next, H&E and immunofluorescence staining was performed to determine if the data collected between the first two groups was considered statistically significant. These scientists found that the group with the sciatic injury displayed increased Schwann cell autophagy and the morphologies of some of the Schwann cells were changed from fusiform
to nearly round. The H&E staining showed that the arrangement of nerve fiber bundles were significantly disordered compared to the sham group’s fiber bundles, in part because of the degeneration and disintegration of the myelin sheaths. When the cultures from the sciatic nerve injury group were treated with the adipose-derived stem cells it was noted that these exosomes inhibited autophagy while promoting the viability of damaged Schwann cells through the release of miRNA-26b. To further investigate and confirm findings, the experiment was repeated but instead of euthanizing the rat, they observed the recovery after being treated with adipose-derived stem cell
exosomes containing miRNA-26b. Eight weeks after treatment the nerve fiber bundles became more organized and tidy. It was also seen that the myelin was structurally intact and increased in number and thickness. This suggests that adipose-derived stem cell exosomes also promote myelin regeneration.
Since the experiment was conducted on a live specimen, and the results were non- conflicting, next steps should be taken to further the advancement on reversing myelindegeneration and the promotion of regeneration. This exciting news in the medical world
confirms the benefits of introducing the exosomes into peripheral nerve injury sites and also explains the mechanism by which this process occurs. Finding solutions for neurodegenerative diseases has just become a little less daunting.
[+] References
Swanson, J. W. (2020, May 5). Find out more about demylinating disease like multiple sclerosis. Mayo Clinic. Retrieved April 29, 2022, from https://www.mayoclinic.org/diseases conditions/multiple-sclerosis/expert-answers/demyelinating-disease/faq-20058521
Morrell, P., & Quarles, R. H. (1999). The myelin sheath - basic neurochemistry - NCBI bookshelf. National Library of Medicine. Retrieved April 30, 2022, from https:// www.ncbi.nlm.nih.gov/books/NBK27954/
Gomez-Sanchez, J. A., Carty, L., Iruarrizaga-Lejarreta, M., Palomo-Irigoyen, M., Varela-Rey, M., Griffith, M., Hantke, J., Macias-Camara, N., Azkargorta, M., Aurrekoetxea, I., De Juan, V. G., Jefferies, H. B. J., Aspichueta, P., Elortza, F., Aransay, A. M., Martínez Chantar, M. L., Baas, F., Mato, J. M., Mirsky, R., … Jessen, K. R. (2015, July 6). Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. The Journal of cell biology. Retrieved April 29, 2022, from https:// www.ncbi.nlm.nih.gov/pmc/articles/PMC4494002/
Gaudet AD, Popovich PG &Ramer MS. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury.Journal of Neuroinflammation.2011 Available from https://jneuroinflammation.biomedcentral.com/articles/ 10.1186/1742-2094-8-110
Yin, G., Yu, B., Liu, C., Lin, Y., Xie, Z., Hu, Y., & Lin, H. (2021, January 6). Exosomes produced by adipose-derived stem cells inhibit Schwann cells autophagy and promote the regeneration of the myelin sheath. The International Journal of Biochemistry & Cell Biology. Retrieved April 29, 2022, from https://www.sciencedirect.com/science/article/ pii/S1357272521000042
Ding, W., Ni, H., Gao, W., Hou, Y., Melissa, A., Chen, X., et al., 2007. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 282, 4702–4710. https://doi.org/10.1074/jbc.M609267200.
Gu, X., Ding, F., Williams, D.F., 2014. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35, 6143–6156. https://doi.org/10.1016/j. biomaterials.2014.04.064. (5*)
Namikawa, K., Okamoto, T., Suzuki, A., Konishi, H., Kiyama, H., 2006. Pancreatitis- associated protein-iii is a novel macrophage chemoattractant implicated in nerve regeneration. J.Neurosci. 26, 7460–7467. https://doi.org/10.1523/ JNEUROSCI.0023-06.2006.
Jessen, K.R., Mirsky, R., Lloyd, A.C., 2015. Schwann cells: development and role in nerve repair. Cold Spring Harbor Perspect. Biol. 7 https://doi.org/10.1101/cshperspect. a020487 a020487.
Wong, Y.K., Zhang, J., Hua, Z.C., Lin, Q., Shen, H.M., Wang, J., 2017. Recent advances in quantitative and chemical proteomics for autophagy studies. Autophagy 13, 1–15. https:// doi.org/10.1080/15548627.2017.1313944.
Koch, J.C., Kno ̈ferle, J., To ̈nges, L., Ostendorf, T., Ba ̈hr, M., Lingor, P., 2010. Acute axonal degeneration in vivo is attenuated by inhibition of autophagy in a calcium- dependent manner. Autophagy 6, 658–659. https://doi.org/10.4161/ auto.6.5.12188.
Madduri, S., Gander, B., 2010. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 15, 93–103. https://doi.org/ 10.1111/ j.1529-8027.2010.00257.x.