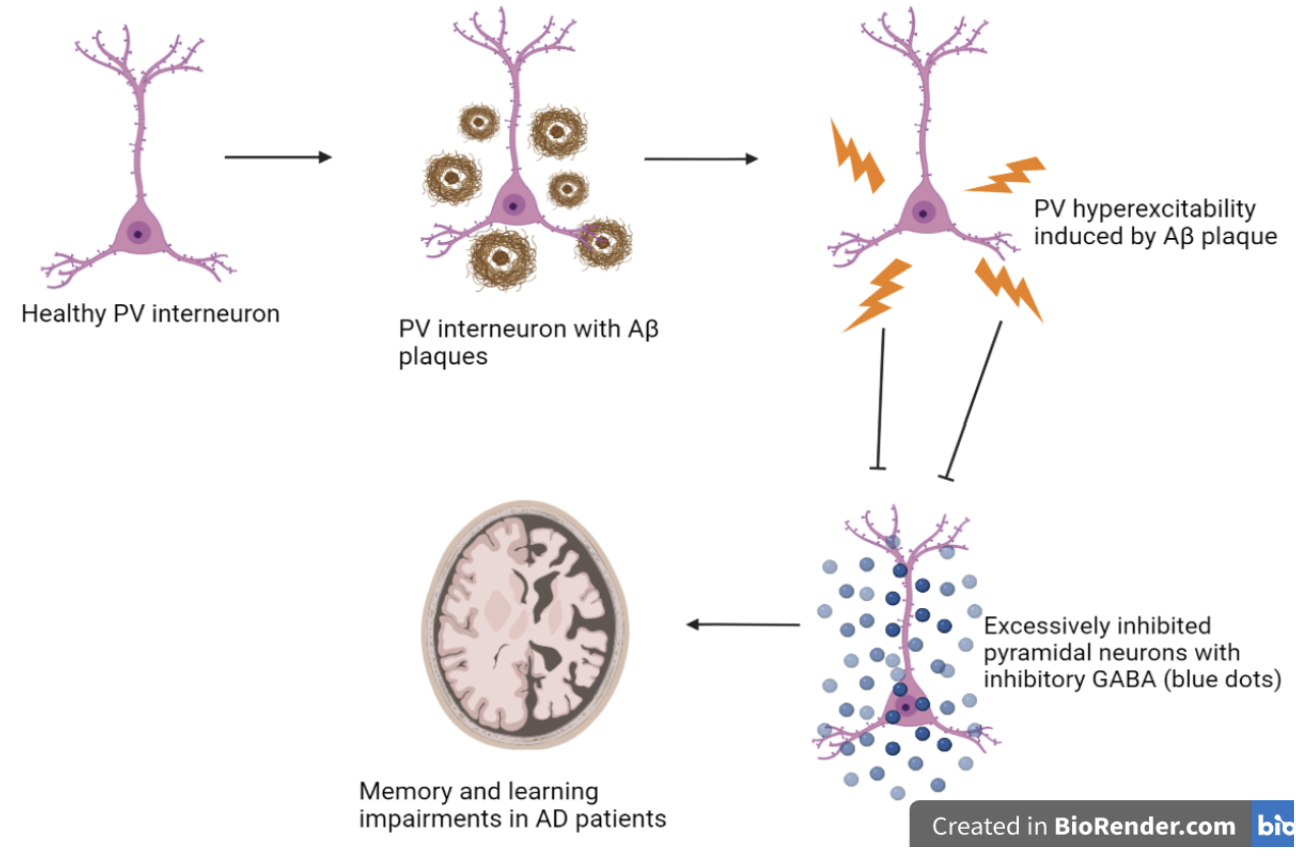
Hyperexcitability of PV interneurons’ effects on memory and learning in AD
In a recent study, hippocampal inhibitory parvalbumin (PV) interneurons were investigated in relation to neuron network dysfunction and memory impairments in Alzheimer's disease (AD). This research suggests early treatment innervation is sufficient to prevent early amyloid-beta-induced hyperexcitability of interneurons, leading to spared memory deficiency in mice models expressing build-up amyloid deposits (APP/PS1 mice). Additionally, this study mentions early intervening treatment results in long-term memory improvement, increased hippocampal network activity, reduced amyloid plaque deposition, and delayed AD progression. This study investigates a novel approach in AD research by looking at the effects of inhibitory network dysfunction in AD pathogenesis allowing for a more diverse research study of AD increasing the possibility of effective therapeutics (Hijazi et al., 2020).
Author: Virginia Buracioc
Neurophysiology
Introduction:
Extensive AD research investigated an abnormal increase in excitatory neuronal activity (enhanced glutamatergic transmission) with AD pathogenesis correlated with a higher prevalence of seizures in AD patients. Recently, however, studies have looked at the effects of the inhibitory network dysfunction on the pathogenesis of AD. PV neurons are GABAergic inhibitory interneurons present in the hippocampus that deliver inhibitory signals to excitatory neurons, predominantly in the hippocampus and throughout the brain (Klausberger et al., 2008). In a paper published by Sara Hijazi, it is mentioned that potential early treatment administration interferences with PV interneurons' hyperexcitability, thus, restoring PV's innate inhibitory function and memory deficits in mice models with abnormal amyloid build up. Experimenters found that PV interneuron function restoration improves hippocampal memory, and network function reduces Aβ levels and delays amyloid plaque formation. This study confirms hyperexcitability of PV interneurons between 15-17 weeks of age in APP/PS1 mice, leading to failure of hippocampal inhibitory function and impairments in memory and learning at 24 weeks. Additionally, the results confirm a correlation between hyperexcitability of PV and inhibitory activity in hippocampal CA1 neurons that leads to memory and learning impairments in AD mice models (Hijazi et al., 2020).
Furthermore, PV interneurons maintain unique cellular processes highly involved in the brain’s neuronal network. Through molecular downstream signaling, PV transmission leads to alteration and changes in cognitive functions (Nahar et al., 2021). Studies mention PVs' crucial role in regulating and balancing excitatory-inhibitory signaling, thus, controlling GABAergic disinhibition between circuitries, an essential process for proper brain function (Nahar et al., 2021). Although the exact PV interneuron behavior and contribution to AD remains unclear, research (Tukker et al., 2007) claims PV interneuron’s crucial role in regulating oscillatory network activity and plasticity in behavioral learning. Extensive research has been performed on pyramidal neurons and their role in AD excitotoxicity has been observed, however this study tests a potential hypothesis that can aid in further understanding AND mechanisms from the perspective of inhibitory interneurons in the hippocampus. Therefore, this new research will provide a clearer understanding of the AD mechanism aiding researchers in developing a therapeutic that is effective and efficient in targeting AD initiation, development, and progression. Previous research has investigated the amyloid cascade hypothesis and its role in AD with little regard to inhibitory cells such as PV interneurons in the brain. This study will fill in the gap in the extensive research on the effects of amyloid-beta toxicity on hippocampal PV interneurons and how hyperexcitability of PV cells affect other principal neurons in the hippocampus, the memory house of the brain.
Methods:
In this study, researchers mimicked the progression and physiology of human AD in mice by introducing and overexpressing mutant forms of amyloid precursor protein (APP) and presenilin (PS) to result in a mouse model (APP/PS1) that represents the Aβ behavior of familial AD cases (Trinchese et al., 2004). mCherry virus injections and whole-cell patch-clamp recordings were performed to observe transmitter(excitatory/inhibitory) action in synaptic transmission in adult models versus control mice. PV excitability effects on hippocampal inhibitory transmission were tested by recording spontaneous inhibitory postsynaptic currents (sIPSCs). To test the effects of high Aβ levels on PV hyperexcitability, hippocampal slices of wild type mice were exposed to a mixture of Aβ peptides followed by whole-cell patch-clamp recordings. To test the role of Aβ plaques in AD mice models, BACE1 inhibition (NB-360) was used to inhibit Aβ production. The experiment further progressed with a prolonged chemo-genetic inhibition treatment that further observed PV interneuron effect in spatial learning and memory. Additionally, optogenetic entrainment was used to restore gamma oscillations and reduce Aβ levels in APP/PS1 hippocampal mice (Hijazi et al., 2020).
Results:
When the experimenters tested the effects of Aβ levels on interneuron excitability by using a BACE1 inhibitor in vivo, researchers observed diminished Aβ levels due to a decrease in interneuron excitability. Thus, further claiming an early increase in PV interneuron hyperactivity in amyloid mice directly correlates with increased levels of Aβ. Additionally, with the help of the BACE1 inhibitor, researchers looked at the long-term beneficial effects of chemo-genetic PV cell inhibition on learning, memory, and properties of the hippocampal network. The results claim no learning or memory impairments were recorded in APP/PS1 mice exposed to the chemo-genetic treatment several weeks after treatment (8 weeks) at 23-25 weeks of age. Furthermore, additional studies (Busche et al., 2015) (Verret et al.,2012) observed redeemed cognitive deficits, restored long-term neuronal hippocampal function, enhanced inhibitory synaptic transmission function, and restoration of hyperexcitability of pyramidal neurons in AD mice after administering PV cell inhibition treatment. Different studies (Palop et al., 2007) additionally (Siskova et al., 2014) claim beneficial effects such as cognitive restoration of intervening treatment when administered over a long-term in AD model mice
Researchers further studied varying PV interneuron activity effects on inhibitory transmission and neural deficits in APP/PS1 mice. When mice were exposed to a chemo-genetic inhibitor during 15-17 weeks of life, experimenters noted improved learning and memory in APP/PS1 mice, claiming the negative role of hyperexcitable PV interneurons in cognitive deficits. Furthermore, along with previous studies (Donato et al., 2013), this research confirms the crucial, precise, and controlled activity of the hippocampal PV interneuron to minimize impairments in memory learning and retrieval. The experimenters followed with a CNO prolonged PV interneuron inhibition treatment in APP/PS1 mice that showed restoration of physiological properties and improved memory deficits in APP/PS1 models, claiming treatment prevents PV cell excitability (Hijazi et al., 2020).
In a study conducted by Iaccarino et al., 2016 observing PV's prolonged intervention on amyloid protein in AD models, findings confirm optogenetic entrainment results in significant restoration of gamma oscillations and reduction of Aβ levels in the hippocampus area. Similar findings of this study suggest significantly decreased amyloid plaques in APP/PS1 mice due to PV interneuron inhibition. Research further claims the role of microglia activation in Aβ clearance. Thus, early PV interneuron restoration leads to a decrease in the possibility of Aβ accumulation. Although ongoing studies claim the effectiveness of positive modulators in GABAergic transmission in restoring memory loss and network function, this current study suggests memory and hippocampal network function can be restored when hyperactive PV interneurons are targeted early in the disease process (Hijazi et al., 2020).
Conclusion:
In this study, researchers observed the behavior of PV interneurons in (APP/PS1) AD mice models. Despite the complexity and conjugated process of AD network progression, research confirms multiple potential interneuron behavior in AD progression. Besides interneuron behavior, neuronal and PV interneuron hyperexcitability and dysfunction play a role in AD initiation and progression. Additionally, despite AD network imbalance and complexity, the findings of this study show that hyper-excited PV interneurons display a harmful and dysfunctional role in the early life of AD (APP/PS1) mice models. Thus, the study suggests additional research on fast-spiking PV interneurons to observe its potential contributing risk factor in AD human patients. Lastly, the study suggests PV interneuron hyperexcitability as an early biomarker to observe oscillatory network function in individuals vulnerable to developing AD (Hijazi et al., 2020). This study will aid in filling in the gap present in the extensive research performed on the amyloid hypothesis in AD and switch focus on the role of inhibitory networks in the progression of AD.
[+] References
Hijazi, S., Heistek, T.S., Scheltens, P. et al. Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease. Mol Psychiatry 25, 3380–3398 (2020). https://doi.org/10.1038/s41380-019-0483-4
Busche MA, Kekus M, Adelsberger H, Noda T, Forstl H, Nelken I, et al. Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat Neurosci. 2015;18:1623–30.
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711.
Siskova Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T, et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron. 2014;84:1023–33.
Frere S, Slutsky I. Alzheimer’s disease: from firing instability to homeostasis network collapse. Neuron. 2018;97:32–58.
Styr B, Slutsky I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat Neurosci. 2018;21:463–73.
Trinchese, F., Liu, S., Battaglia, F., Walter, S., Mathews, P.M. and Arancio, O. (2004), Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol., 55: 801-814. https://doi.org/10.1002/ana.20101
Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–6.
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–5.
Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008 Jul 4;321(5885):53-7. DOI: 10.1126/science.1149381. PMID: 18599766; PMCID: PMC4487503.
Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–9.
Nahar, L., Delacroix, B. M., & Nam, H. W. (2021). The Role of Parvalbumin Interneurons in Neurotransmitter Balance and Neurological Disease. Frontiers in psychiatry, 12, 679960. https://doi.org/10.3389/fpsyt.2021.679960
[+] Other Work By Virginia Buracioc
Natural Killer Cells in Amyotrophic Lateral Sclerosis
Neuroanatomy
In this study the subgroup of familial ALS (fALS) disorder was investigated by studying the presence of the mutant superoxide dismutase 1 (SOD1) gene with respect to Natural killer (NK) cells in the death of motor neurons (11). This study included both ALS patients and SOD1 mice and concluded that the presence of NK cells was correlated with ALS symptoms and ALS markers such as damaged microglia and the suppression of protective cells.
Reducing Inflammation by Targeting Lymphocyte Expression in Relapse Remitting Multiple Sclerosis
Neuroscience In Review