Reward and Aversion, the Two Not so Separate Pathways
Previously it was thought that the brain had separate reward and aversion pathways, but a recent study suggests that reward and aversion might be encoded by both pathways.
Author: Meri Kokki
Neurophysiology
Reward and aversion are important players in many adaptive behaviors, such as drug usage. Both, reward, and aversion, are encoded in response to neurotransmitter dopamine, but through different pathways, depending on the dopamine receptors found on the cells in these pathways. However, recently there have been indications that there might be some overlap in these pathways. In a paper published in Molecular Psychiatry, Soares-Cunha et al.1 hypothesized that instead of being separate pathways, the response depends on the pattern of stimulation. Using genetically modified mice, they found that, depending on the length of the stimulus, both pathways can indeed encode for reward and aversion. Dysfunction of the reward circuit is present at addiction, and depression, and even though further studies are needed, this is a first step in further understanding the complexity of the reward circuit.
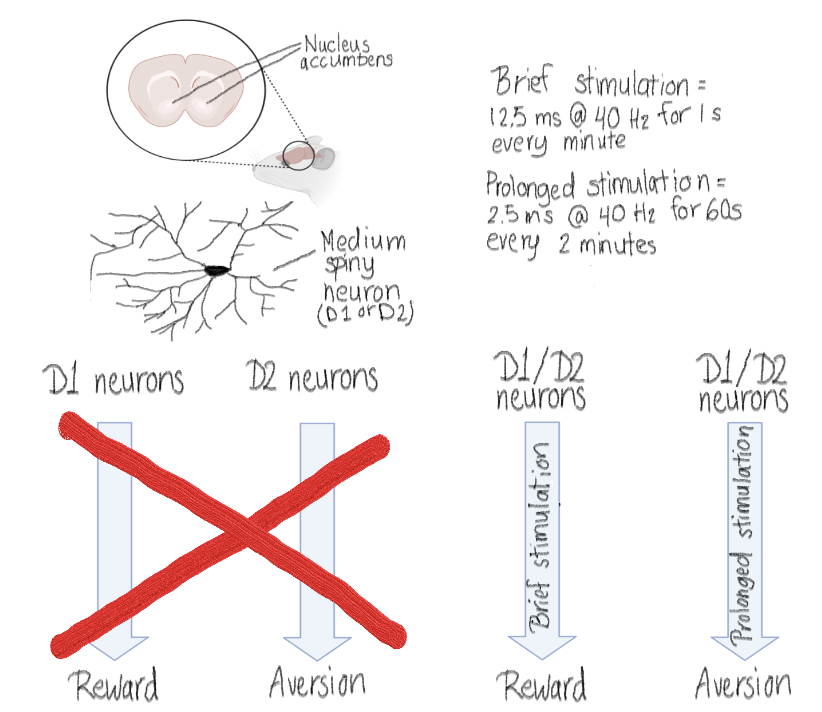
The ventral tegmental area is an important part of the reward pathway, and ~60% of the ventral tegmental area neurons release dopamine.2 Nucleus accumbens, part of the striatum, receives dopaminergic inputs from the ventral tegmental area.3 Ninety to ninety-five percent of the neurons in striatum, and therefore in the nucleus accumbens, are medium spiny neurons.4 There are two subtypes of the medium spiny neurons, D1 and D2, named after the different dopamine receptors they express.4 Until recently, the D1 pathway of the nucleus accumbens is thought to be encoding reward, and D2 pathway aversion.5 However, in last few years there have been evidence that D2 pathway can also encode reward. 6,7
Soares-Cunha et al. used optogenetics to test the stimulation pattern of the D1 and D2 neurons. In optogenetics, ion channels (such as channelrhodopsins) from microbial origins can be introduced to a population of neurons and these channels can then be activated with light leading to activation (or inhibition) of the said neurons.8 The mice were modified so that either their D1 neurons or D2 neurons expressed channelrhodopsin 2 receptors and therefore could be stimulated by light via the optical fiber implanted to their heads. Then the researchers used either brief stimulation, or prolonged stimulation of the neurons, and observed the behavior of the mice. Both stimuli used 12.5 millisecond pulses of light at 40 Hertz, but the brief stimulation lasted only 1 second (given every one minute), while the prolonged stimulation lasted for 60 seconds (given every two minutes). The behavior the researchers measured was conditioned place preference, in which the mice could decide in what chamber they want to spend time in. There were two options, one called ON chamber and one called OFF chamber. In the ON chamber the mice received the stimulus (either the brief or prolonged depending on what they tested at the time) until they went to the OFF chamber. More time in the ON chamber suggests that the stimulus was rewarding, and less time that it was aversive. The researcher also tested the conditioned place preference with cocaine. In this experiment the mice received cocaine in addition to the stimulus in the ON chamber, and no stimulus with saline in the off chamber.
The main result of the study was that reward and aversion are encoded by both pathways (Figure 1). When either pathway was stimulated briefly, the mice spent more time in the ON chamber than in the OFF chamber. The opposite was true with prolonged stimulation. Brief stimulation also increased preference for cocaine regardless of the pathway. However, with the prolonged stimulation, the stimulation of the D1 pathway had no effect on the cocaine preference, but prolonged stimulation of the D2 pathway decreased the cocaine preference.
The dopamine coming from the ventral tegmental area to nucleus accumbens is important for the goal-directed behavior9, and dysfunction of this dopamine pathway is present in depression and addiction.10,11 Usually the neurons in the nucleus accumbens fire with low frequency, and the authors note that the prolonged stimulation used in the study might elicit unnatural response. However, they also mention that there have been prolonged excitation and inhibition of a fraction of nucleus accumbens neurons in animal studies with cocaine self-administration. All in all, it is interesting that reward and aversion can be encoded by both D1 and D2 pathways, contradicting the previous idea of them being separate pathways. It is important to study this further since the dysfunction of the reward pathway is seen in both depression and addiction, both of which are very prevalent conditions. Therefore, increased understanding of the complexity of the reward pathway could help to better understand depression and addiction.
[+] References
Soares-Cunha, C., de Vasconcelos, N., Coimbra, B., Domingues, A. V., Silva, J. M., Loureiro-Campos, E., Gaspar, R., Sotiropoulos, I., Sousa, N., & Rodrigues, A. J. (2020). Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Molecular psychiatry, 25(12), 3241–3255.
Bouarab, C., Thompson, B., & Polter, A. M. (2019). VTA GABA neurons at the interface of stress and reward. Frontiers in neural circuits, 78.
Qi, J., Zhang, S., Wang, H. L., Barker, D. J., Miranda-Barrientos, J., & Morales, M. (2016). VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nature neuroscience, 19(5), 725–733.
Lobo, M. K., & Nestler, E. J. (2011). The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Frontiers in neuroanatomy, 5, 41.
Lin, Y. H., Yamahashi, Y., Kuroda, K., Faruk, M. O., Zhang, X., Yamada, K., ... & Kaibuchi, K. (2021). Accumbal D2R-medium spiny neurons regulate aversive behaviors through PKA-Rap1 pathway. Neurochemistry International, 143, 104935.
Natsubori, A., Tsutsui-Kimura, I., Nishida, H., Bouchekioua, Y., Sekiya, H., Uchigashima, M., Watanabe, M., de Kerchove d'Exaerde, A., Mimura, M., Takata, N., & Tanaka, K. F. (2017). Ventrolateral Striatal Medium Spiny Neurons Positively Regulate Food-Incentive, Goal-Directed Behavior Independently of D1 and D2 Selectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience, 37(10), 2723–2733.
Soares-Cunha, C., Coimbra, B., David-Pereira, A., Borges, S., Pinto, L., Costa, P., Sousa, N., & Rodrigues, A. J. (2016). Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nature communications, 7, 11829.
Fenno, L., Yizhar, O., & Deisseroth, K. (2011). The development and application of optogenetics. Annual review of neuroscience, 34, 389–412.
Goto, Y., & Grace, A. A. (2005). Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature neuroscience, 8(6), 805-812.
Pizzagalli, D. A., Holmes, A. J., Dillon, D. G., Goetz, E. L., Birk, J. L., Bogdan, R., Dougherty, D. D., Iosifescu, D. V., Rauch, S. L., & Fava, M. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American journal of psychiatry, 166(6), 702–710.
Boudreau, A. C., & Wolf, M. E. (2005). Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience, 25(40), 9144–9151.