New genetically modified mouse model for Parkinson’s disease
A new study shows that a new type of genetically modified mouse could open up new therapies for Parkinson’s disease
Author: Olivia Molano
Neurophysiology
Abstract
Parkinson's Disease (PD) is one of the most common progressive neurodegenerative disorders. About 60,000 Americans are diagnosed with PD every year (Marras et al., 2018). Parkinson’s is a movement disorder that additionally has cognitive impairments. Parkinson's disease is caused by the degradation of dopaminergic neurons located in the substantia nigra. Additionally, the formation of Lewy bodies which are intracellular aggregates of misfolded alpha synuclein proteins (αSNCA) (Sun et al., 2013). Currently, to investigate the mechanism associated with αSNCA and PD there are mouse models that have different administration routes of pαSNCA to increase αSNCA aggregation. However, most of these methods are invasive and sometimes not effective (Lin et al., 2022). In a paper recently published in Pharmaceutics, scientists talk about a new administration route called ultrasound-targeted microbubble destruction (UTMD) that generates the phenotype of PD through αSNCA CNS gene delivery. This study shows that UTMD is an effective new method that has great possibilities and potential as a new therapeutic strategy for Parkinson’s disease.
Background
As mentioned, Parkinson's Disease is one of the most common progressive neurodegenerative disorders. About 60,000 Americans are diagnosed with PD every year (Marras et al., 2018). Parkinson’s is a movement disorder that additionally has cognitive impairments. In the earlier stages of PD patients experience tremors, bradykinesia, and motor impairments (Giasson & Lee, 2001). As PD progresses nonmotor symptoms come into play like dementia, depression, anxiety and psychosis (Park & Stacy, 2009). Parkinson's disease is caused by the degradation of dopaminergic neurons, the main source of dopamine, located in the substantia nigra (Chinta & Andersen, 2005). Additionally, PD is characterized by the formation of Lewy bodies which are intracellular aggregates of misfolded alpha synuclein proteins (αSNCA) (Sun et al., 2013). The accumulation and aggregation of αSNCA have been associated with the neurodegradation of dopaminergic neurons (Lin et al., 2022; Rodríguez-Losada et al., 2020).
A pathological hallmark of Parkinson's disease is an accumulation of αSNCA Lewy bodies (Lin et al., 2022). This hallmark is used in research to find a good PD model. Currently, to investigate the mechanism associated with αSNCA and PD there are mouse models that have different administration routes to increase αSNCA aggregation (Lin et al., 2022). However, most of these methods are invasive and sometimes not effective (Lin et al., 2022). In this study, it talks about a new administration route called ultrasound-targeted microbubble destruction (UTMD) that generates the phenotype of PD through αSNCA CNS gene delivery. UTMD is a type of ultrasound therapy where low frequency powered ultrasound is combined with micro bubbles in order to trigger Cavitation (Wischhusen & Padilla, 2019). Cavitation is the process of gas bubbles to permeabilize biological barriers. This method is helpful to open the blood brain barrier and allows genes to target central nervous system sites (Wischhusen & Padilla, 2019). In this experiment they used UTMD delivery of liposome-encapsulated αSNCA (pαSNCA) genes and compared it to other PD models.
Methods
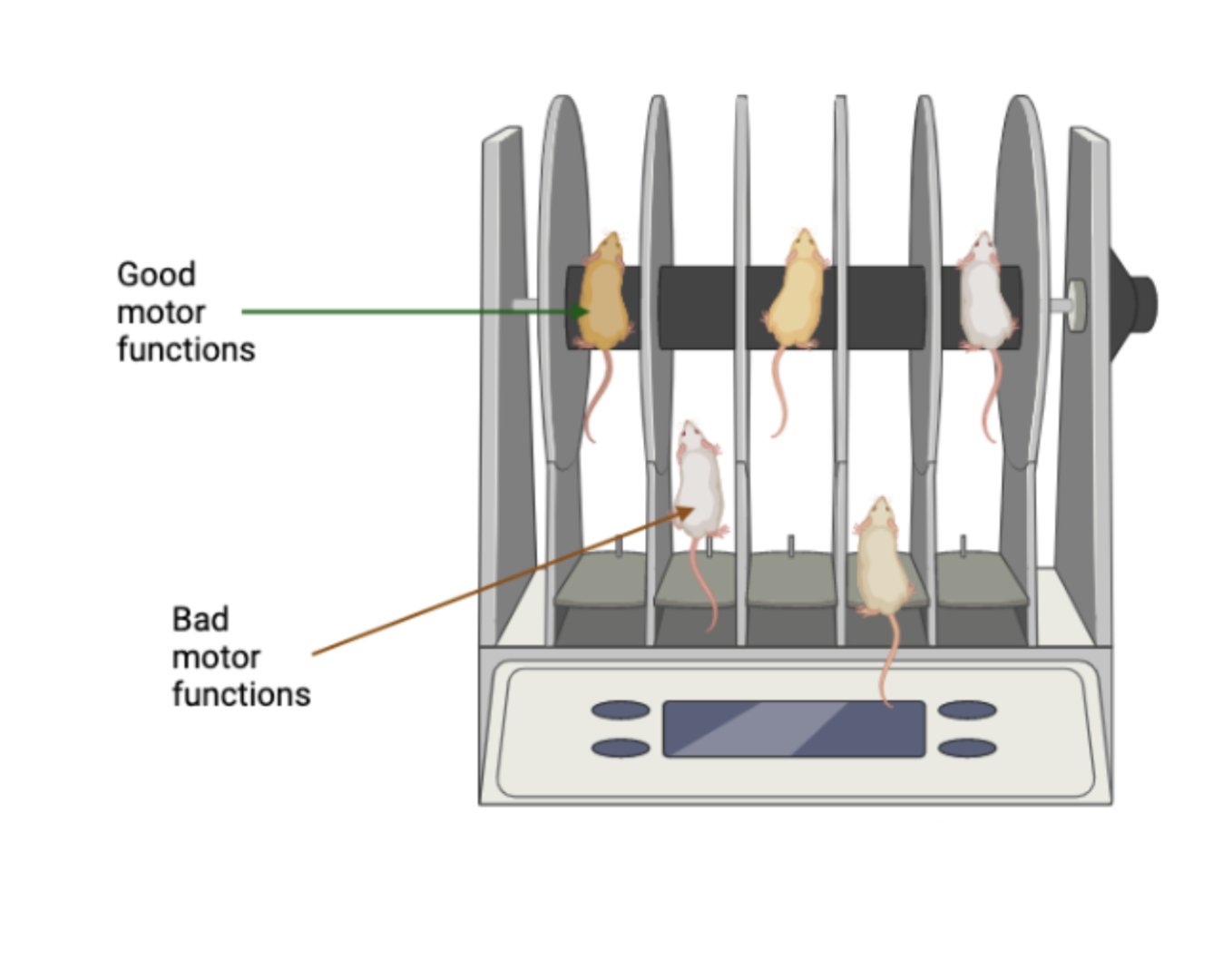
Throughout the study they did three different types of models. One with MPTP injection, pαSNCA intracerebral (IC) injection and pαSNCA intravenous infusion (IV) with UTMD (Lin et al., 2022). MPTP is a gold standard PD mouse model and IC injection is also used in the field of PD (Meredith & Rademacher, 2011; Lin et al., 2022). In the first experiment they set out to find out if each model had a loss of dopaminergic neurons and increased neuroinflammation which is a key player in motor symptoms during PD progression. They examined deficits in motor function with a rotarod device for all three models (Lin et al., 2022). This device has mice on a tube and see how long they can stand on that tube. Specifically this test measures balance, grip strength and most importantly motor coordination which is impacted for patients with PD (Figure 1.) (Deacon, 2013). They performed a high-performance liquid chromatography (HPLC) to help determine the neurotransmitter levels of dopaminergic neurons (Lin et al., 2022). Their next experiment was evaluating the efficacy of targeted transfection by intravenous infusion using bioluminescent imaging (Lin et al., 2022). Next, they used H&E staining which is used to observe the morphological changes in the substantia nigra (Fischer et al., 2008). The last experiment was using fluorescent labeled immunohistochemistry substantia nigra based staining to see any pathological changes in the substantia nigra (Lin et al., 2022). Fluorescent labeled immunohistochemistry is used to detect the location of proteins and antigens. They are able to detect the pathological changes using a fluorescent dye (Zaqout et al., 2020).
Results
When comparing the three different administration routes to induce PD on a mouse model they had found that with the administration via IV in conjunction with UTMD it provides an ideal αSNCA based PD model (Lin et al., 2022). The gold standard model of MPTP can barely contain any aggregate formation in the brain of the mice and dopamine neurodegreadtion does not seem to be stable which is not consistent with PD. Additionally, MPTP can be invasive and cause stress upon the mouse. They found that the UTMD method is ideal as it is a noninvasive procedure and can offer fast phenotype expression along with phenotype promoting stability (Lin et al., 2022). When using this method, it had induced αSNCA expression which caused dopamine neuronal loss in the substantia nigra which aligns with PD phenotype (Lin et al., 2022).
Final paragraph
Overall, the implication of this paper is that there is a new and improved method for inducing a PD mouse model. This method seems to be less invasive of a procedure compared to the other administration routes for a PD model like intracerebral injection or the MPTP injection. This study shows that UTMD is an effective new method that has great possibilities in finding new therapeutic strategies for Parkinson’s disease.
[+] References
Chinta, S. J., & Andersen, J. K. (2005). Dopaminergic neurons. The International Journal of Biochemistry & Cell Biology, 37(5), 942–946. https://doi.org/10.1016/j.biocel.2004.09.009
Deacon, R. M. J. (2013). Measuring Motor Coordination in Mice. Journal of Visualized Experiments : JoVE, 75, 2609. https://doi.org/10.3791/2609
Fischer, A. H., Jacobson, K. A., Rose, J., & Zeller, R. (2008). Hematoxylin and eosin staining of tissue and cell sections. CSH Protocols, 2008, pdb.prot4986. https://doi.org/10.1101/pdb.prot4986
Giasson, B. I., & Lee, V. M. (2001). Parkin and the molecular pathways of Parkinson’s disease. Neuron, 31(6), 885–888. https://doi.org/10.1016/s0896-6273(01)00439-1
Lin, C.-Y., Huang, C.-Y., Chen, C.-M., & Liu, H.-L. (2022). Focused Ultrasound-Induced Blood–Brain Barrier Opening Enhanced α-Synuclein Expression in Mice for Modeling Parkinson’s Disease. Pharmaceutics, 14(2), 444. https://doi.org/10.3390/pharmaceutics14020444
Marras, C., Beck, J. C., Bower, J. H., Roberts, E., Ritz, B., Ross, G. W., Abbott, R. D., Savica, R., Van Den Eeden, S. K., Willis, A. W., Tanner, C. M., & Parkinson’s Foundation P4 Group. (2018). Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Disease, 4, 21. https://doi.org/10.1038/s41531-018-0058-0
Meredith, G. E., & Rademacher, D. J. (2011). MPTP Mouse Models of Parkinson’s Disease: An Update. Journal of Parkinson’s Disease, 1(1), 19–33. https://doi.org/10.3233/JPD-2011-11023
Park, A., & Stacy, M. (2009). Non-motor symptoms in Parkinson’s disease. Journal of Neurology, 256 Suppl 3, 293–298. https://doi.org/10.1007/s00415-009-5240-1
Rodríguez-Losada, N., de la Rosa, J., Larriva, M., Wendelbo, R., Aguirre, J. A., Castresana, J. S., & Ballaz, S. J. (2020). Overexpression of alpha-synuclein promotes both cell proliferation and cell toxicity in human SH-SY5Y neuroblastoma cells. Journal of Advanced Research, 23, 37–45. https://doi.org/10.1016/j.jare.2020.01.009
Sun, X., Liu, J., Crary, J. F., Malagelada, C., Sulzer, D., Greene, L. A., & Levy, O. A. (2013). ATF4 Protects Against Neuronal Death in Cellular Parkinson’s Disease Models by Maintaining Levels of Parkin. The Journal of Neuroscience, 33(6), 2398–2407. https://doi.org/10.1523/JNEUROSCI.2292-12.2013
Wischhusen, J., & Padilla, F. (2019). Ultrasound-Targeted Microbubble Destruction (UTMD) for Localized Drug Delivery into Tumor Tissue. IRBM, 40(1), 10–15. https://doi.org/10.1016/j.irbm.2018.11.005
Zaqout, S., Becker, L.-L., & Kaindl, A. M. (2020). Immunofluorescence Staining of Paraffin Sections Step by Step. Frontiers in Neuroanatomy, 14, 582218. https://doi.org/10.3389/fnana.2020.582218
[+] Other Work By Olivia Molano
Finding the Supreme Mouse Model for Anxiety Disorders.
Neuroanatomy
129s1/Svlmj mice show sleep pattern similar to those with anxiety and PTSD.
Aminoglycoside and Cisplatin Induced Ototoxicity
Neuroscience In Review