Alcohol Has Opposing Effects on the Brains of Men and Women
A recent study found male and female rats experience opposing modifications to the activity of their inhibition nerve cells after alcohol use.
Author: Keziah Nguyen
Neurophysiology
Introduction
Drinking is a popular social activity amongst all age groups with more than 85% of people ages 18 and older having reported consuming alcohol at some point in their lives (NIAAA, 2022). Excessive consumption can lead to alcohol use disorder (AUD). AUD is comorbid with other substance use disorders, major depressive disorder, and bipolar disorders (Grant et al., 2015). It can severely impair daily functions because AUD causes impaired working memory and decreased control of inhibitions (Bokura et al., 2001). Thus, with its prevalent use amongst a wide demographic, it is important to understand the effects of long-term alcohol use. Currently, many studies have investigated the effect of alcohol on prefrontal cortex excitatory nerve cells as they serve as the main source of activity in the prefrontal cortex, an area of the brain responsible for higher level control and decision-making (Price & McCool, 2022, Delatour et al., 2020, McGinnis et al., 2020). In contrast, the field lacks studies investigating the effect of alcohol upon inhibitory nerve cells in this same region. In 2020, Benjamin Hughes and colleagues investigated the effects of long-term alcohol exposure in the prefrontal cortex of mice specifically upon inhibitory nerve cells called Martinotti cells. The researchers measured electrical activity in both male and female mice brains after long-term alcohol consumption. They found sex differences in both baseline Martinotti cell activity and subsequently after alcohol exposure.
Background
The prefrontal cortex is connected to many other regions of the brain to order, time, and execute activity (Abernathy et al., 2010). Glutamate, an excitatory signaling molecule, is the primary regulator of changes within the prefrontal cortex and these signals are modified under alcohol exposure (Chandler, 2003). There is evidence that AUD causes impaired working memory and reduced control of inhibitions within the prefrontal cortex, both of whose effects are mediated by glutamate (Bokura et al., 2001). This activity is also influenced by GABA, an inhibitory signaling molecule. Under normal conditions, nerve cells releasing GABA, called interneurons, refine the activity of networks of these glutamatergic nerve cells (Abernathy et al., 2010). However, not much is understood about the modification of interneurons under long-term alcohol exposure.
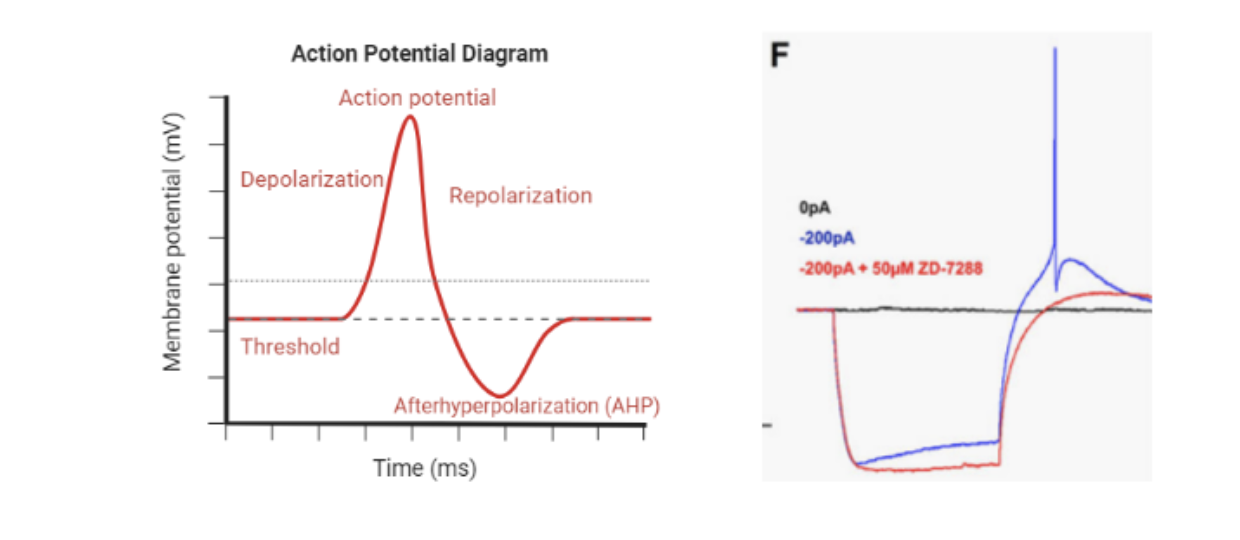
Martinotti cells target the dendrites of excitatory nerve cells in the prefrontal cortex to provide local inhibition (Silberberg & Markram, 2007). These cells often activate again immediately after being activated (depolarized) because of the presence of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. HCN channels are activated during the afterhyperpolarization phase of an action potential to allow sodium and potassium to flow into the cell (Benarroch, 2013). These positively charged ions increase the membrane potential towards the activation threshold leading to another action potential. Thus, Hughes and colleagues sought to investigate the effect of alcohol upon Martinotti cells and their HCN channel activity.
Methods
In this study, Sprague-Dawley male and female rats were injected with a virus to drive expression of green fluorescent protein in prefrontal cortical Martinotti cells to visualize the cells. The rats were divided into two treatment groups: water exposure and alcohol exposure. Then, the rats were euthanized, and the brains were extracted and sliced. Electrophysiology techniques were used to record and compare the activity of Martinotti cells in rats exposed to water and rats exposed to alcohol. Series of current injections were applied to record the response in the cells under normal conditions and under the presence of HCN channel inhibition. Also, Western blotting, a technique used to measure protein content, was used to quantify the expression of HCN channels in Martinotti cells of male and female rats. HCN channels are a hallmark of Martinotti cell physiology and function.
Results
There was a sex difference in Martinotti cell activity during baseline and after alcohol exposure. Female rats had 5 times the expression of HCN channels compared to males at baseline. This difference in expression was maintained during alcohol exposure, but there was no effect of alcohol upon HCN channel expression in either sex. However, alcohol did affect the function of HCN channels in a sex-dependent manner. Alcohol-exposed males had an increased voltage rebound induced by HCN channels compared to water-exposed males. In alcohol-exposed females, there was a reduction in the voltage rebound compared to water-exposed females. Furthermore, in male rats, alcohol induced an enhancement of Martinotti excitability while in female rats, alcohol reduced Martinotti excitability measured by the half maximal effective concentration. Alcohol-exposed males required less GABA than water-exposed males for the current peak to reach 50% of its max amplitude. In contrast, alcohol-exposed females required more GABA than water-exposed females.
Conclusion
Long-term alcohol exposure results in more inhibition in males which may serve to increase control over overexcited glutamate-releasing nerve cells corroborating prior findings (Bokura et al., 2001). However, in female rats, alcohol exposure decreased inhibitions. This contradictory finding warrants further research to elucidate the function of decreased inhibition in female rats. There was also a difference in HCN channel expression between sexes with female rats displaying 5 times the amount of HCN channels than male rats. Future studies are needed to explain the purpose of this large expression of HCN channels as well. Understanding sex-dependent responses to alcohol exposure is necessary for informing our treatment of AUD. AUD affects 14.5 million Americans with a greater proportion of this population being male AUD Americans (9.0 million), and electrophysiological differences between male and female AUD patients may explain this sex-difference in the number of AUD sufferers (NIAAA, 2022).
[+] References
Abernathy, K., Chandler, L. J., & Woodward, J. J. (2010). Alcohol and the prefrontal cortex. International review of neurobiology, 91, 289–320. https://doi.org/10.1016/S0074-7742(10)91009-X
Benarroch E. E. (2013). HCN channels: function and clinical implications. Neurology, 80(3), 304–310. https://doi.org/10.1212/WNL.0b013e31827dec42 https://doi.org/10.1212/WNL.0b013e31827dec42
Bokura H, Yamaguchi S, and Kobayashi S, Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol, 2001 112(12): p. 2224–32.
Chandler LJ. Ethanol and brain plasticity: Receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol. Ther. 2003; 99:311–326. [PubMed: 12951163]
Delatour, L. C., Yeh, P., & Yeh, H. H. (2020). Prenatal Exposure to Ethanol Alters Synaptic Activity in Layer V/VI Pyramidal Neurons of the Somatosensory Cortex. Cerebral cortex (New York, N.Y. : 1991), 30(3), 1735–1751. https://doi.org/10.1093/cercor/bhz199
Grant, B. F., Goldstein, R. B., Saha, T. D., Chou, S. P., Jung, J., Zhang, H., Pickering, R. P., Ruan, W. J., Smith, S. M., Huang, B., & Hasin, D. S. (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry, 72(8), 757–766. https://doi.org/10.1001/jamapsychiatry.2015.0584
McGinnis, M. M., Parrish, B. C., Chappell, A. M., Alexander, N. J., & McCool, B. A. (2020). Chronic Ethanol Differentially Modulates Glutamate Release from Dorsal and Ventral Prefrontal Cortical Inputs onto Rat Basolateral Amygdala Principal Neurons. eNeuro, 7(2), ENEURO.0132-19.2019. https://doi.org/10.1523/ENEURO.0132-19.2019
Price, M. E., & McCool, B. A. (2022). Chronic Alcohol Dysregulates Glutamatergic Function in the Basolateral Amygdala in a Projection-and Sex-Specific Manner. Frontiers in cellular neuroscience, 16, 857550. https://doi.org/10.3389/fncel.2022.857550
Silberberg, G., & Markram, H. (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron, 53(5), 735–746. https://doi.org/10.1016/j.neuron.2007.02.012
U.S. Department of Health and Human Services. (2022, March). Alcohol Facts and Statistics. National Institute on Alcohol Abuse and Alcoholism. Retrieved May 2, 2022, from https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
[+] Other Work By Keziah Nguyen
Ketamine: Horse Tranquilizer, Human Antidepressant
Neuroanatomy
The study examines how ketamine is able to quickly block lateral habenula activity to alleviate depressive symptoms. Previously, it was known that ketamine was an effective rapidly acting antidepressant, but the way it worked was not understood.
Exercise Mediates Effects Upon Opioid Dependence and Withdrawal
Neuroscience In Review