The Master Adapter to any Environment: How Special Types of Neurons in the Hippocampus of the Brain will Change Their Physiology to Maintain Stable Excitability.
The article, Homeostatic regulation of axonal Kv1.1 channels accounts for both synaptic and intrinsic modifications in the hippocampal CA3 circuit, describes how CA3 pyramidal neurons in the hippocampus of the brain will regulate their own excitability levels. This is accomplished by decreasing the number of potassium channels on the neuron’s axon initial segment, which are responsible for lowered excitation level of the cell.
Author: Abigail O'Niel
Neurophysiology
Introduction
The human brain is a dynamic organ with the ability to change the way it behaves in response to stimuli. This concept is referred to as homeostatic plasticity. A neuron can change its shape and connectivity to other neurons is a circuit in response to how often it is receiving messages (Tien & Kerschensteiner, 2018) Typically, higher frequency input onto a neuron increases the likelihood of how it responds. Neurophysiologists call this excitability, and when talking about a singular neuron, it is referred to as intrinsic excitability (Zbili et al., 2021). This ability to change excitability of neurons is a crucial aspect of the brain, because without the ability to change circuits we would not be able to learn new tasks or make new memories (Dunn & Kaczorowski, 2019). Researchers at The Cajal Institute in Madrid sought to explore this phenomenon in the area of the brain associated with memory formation, called the hippocampus. They found that the regulation of synaptic transmission and intrinsic excitability are closely related. By blocking the excitatory synaptic receptors of the CA3 pyramidal neurons in the hippocampus, they discovered an up-regulation of both synaptic transmission and intrinsic excitability of those neurons. They contributed this change to a reduction in a special type of ion channel, called Kv1.1 channels.
Background
The pyramidal cells in the hippocampus undergo experience- and learning-related plasticity in intrinsic properties, specific to the type of cell they are and the type of input they receive (Dunn et al., 2019). The most common types of neurons in the hippocampus are the regular spiking (RS) and burst spiking (BS) neurons, which describe the kind of electrical signals they put out (Zbili et al., 2021). Intrinsic excitability is the electrical excitability of a particular neuron. It is determined by the number and distribution of ion channels and receptors on the neuron (Dunn et al., 2019). The type of ion channels present will set the resting voltage of the cell, called the membrane potential. For a neuron to become excited, it has to have this membrane potential voltage changed to a different level, called threshold. A neuron reaching threshold and becoming excited is called depolarization, meaning it fires off electrical activity (Chen Lui., 2021). A neuron that expresses many sodium channels will be easily excited, while a neuron that expresses more potassium channels will not be as easily excited (Huguenard, 2002). Intrinsic excitability gives the neuron the ability to control its own excitability within a circuit by changing the expression of the channels it expresses (Zbili et al., 2021).
Methods
To perform this experiment, researchers at The Cajal Institute slices of the hippocampus from rat brains. They blocked excitatory input on the CA3 pyramidal network to deprive them of excitatory stimuli by bathing the neurons in a compound called kynurenate. The control groups were given the same stimuli but were not bathed in the compound. To ensure that the changes in activity were in response to the change in Kv1.1 channel expression, the researchers blocked the Kv1.1 channels with a drug called DTX-K and performed the same experiment as before. The researchers recorded the electrical activity of these cells using a technique called paired recording to study communication between individual neurons in small networks. This involves an electrode being attached to two neurons that are in the same network, so the activity from one neuron to the other can be measured (Debanne et al., 2008). To visualize and quantify the expression of Kv1.1 channels, the researchers did an immunostaining technique which colorizes the proteins so they can be seen. With the staining technique they used the protein called Ankyrin G, the protein that literally anchors the ion channels to the neuron, as a control. The purpose of this control was to show that the neuron was still anchoring proteins on itself, but the type of ion channel was either increasing or decreasing regardless.
Results
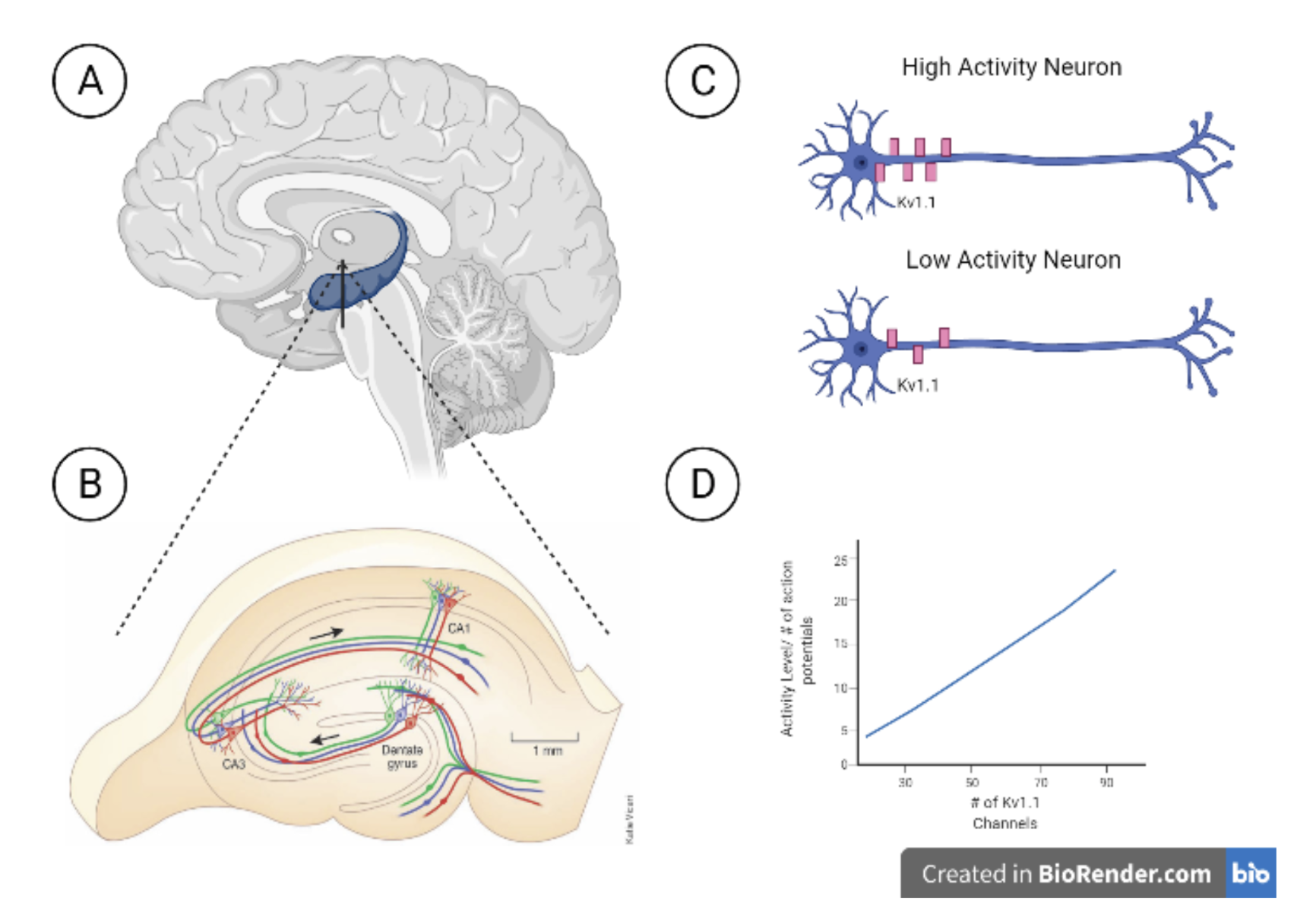
The study by the researchers at The Cajal Institute found that neurons will change the expression of the potassium channels, Kv1.1, to control how excited the neuron could be. Depending on the amount of stimulation the neuron is receiving, it will alter its own genetic make-up to change the number of potassium ion channels found on the body and axon of the cell. They found that a high activity CA3 pyramidal neuron would have many Kv1.1 channels (Figure 1C-D), while a low activity CA3 pyramidal neuron would have fewer Kv1.1 channels (Figure 1C-D). What is interesting about their study is that they found that CA3 pyramidal neurons that started with many Kv1.1 channels experienced a decrease in the number of Kv1.1 channels if they deprived that neuron of excitatory input. Along with this, there was an increase in CA3 pyramidal cell intrinsic excitability in activity-deprived neurons when compared to the controls. They found a direct correlation between the number of stimulating shocks onto a neuron and the number of Kv1.1 channels it expressed.
Conclusion
The significance of this study resides in the fact that the brain loves to inhibit itself. The proper balance of excitation and inhibition of the neurons is crucial to a smoothly operating brain (Giorgi & Marinelli, 2021). Too much excitatory activity in the hippocampus has been linked in previous research to seizures (Lado et al., 2002) and schizophrenia (Wolff et al., 2018). Given the importance of excitability control, the intrinsic ability of CA3 neurons to change their expression of the protein Kv1.1 is a very important concept in the field of neurophysiology. The study by the researchers at The Cajal Institute in Madrid demonstrated that the intrinsic regulation of Kv1.1 channels is an important and effective method for the hippocampus to control its excitability at the level of a single neuron.
[+] References
Chen I, Lui F. Neuroanatomy, Neuron Action Potential. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546639/
D. Debanne et al., Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Protoc. 3, 1559–1568 (2008).
Dunn, A. R., & Kaczorowski, C. C. (2019). Regulation of intrinsic excitability: Roles for learning and memory, aging and alzheimer’s disease, and genetic diversity. Neurobiology of Learning and Memory, 164, 107069. https://doi.org/10.1016/j.nlm.2019.107069
Dunn, A. R., Neuner, S. M., Ding, S., Hope, K. A., O'Connell, K., & Kaczorowski, C. C. (2019). Cell-Type-Specific Changes in Intrinsic Excitability in the Subiculum following Learning and Exposure to Novel Environmental Contexts. eNeuro, 5(6), ENEURO.0484-18.2018. https://doi.org/10.1523/ENEURO.0484-18.2018
Giorgi, C., & Marinelli, S. (2021). Roles and Transcriptional Responses of Inhibitory Neurons in Learning and Memory. Frontiers in molecular neuroscience, 14, 689952. https://doi.org/10.3389/fnmol.2021.689952
Huguenard, J. R. (2002). Sodium channels. Neuron, 33(4), 492–494. https://doi.org/10.1016/s0896-6273(02)00592-5
Lado, F. A., Laureta, E. C., & Moshé, S. L. (2002). Seizure-induced hippocampal damage in the mature and immature brain. Epileptic disorders : international epilepsy journal with videotape, 4(2), 83–97.
Tien, N. W., & Kerschensteiner, D. (2018). Homeostatic plasticity in neural development. Neural development, 13(1), 9. https://doi.org/10.1186/s13064-018-0105-x
Wolff, A.R., Bygrave, A.M., Sanderson, D.J. et al. Optogenetic induction of the schizophrenia-related endophenotype of ventral hippocampal hyperactivity causes rodent correlates of positive and cognitive symptoms. Sci Rep 8, 12871 (2018). https://doi.org/10.1038/s41598-018-31163-5
Zbili, M., Rama, S., Benitez, M. J., Fronzaroli-Molinieres, L., Bialowas, A., Boumedine-Guignon, N., Garrido, J. J., & Debanne, D. (2021). Homeostatic regulation of axonal Kv1.1 channels accounts for both synaptic and intrinsic modifications in the hippocampal CA3 circuit. Proceedings of the National Academy of Sciences of the United States of America, 118(47), e2110601118. https://doi.org/10.1073/pnas.2110601118
N., Garrido, J. J., & Debanne, D. (2021). Homeostatic regulation of Axonal KV1.1 channels accounts for both synaptic and intrinsic modifications in the hippocampal CA3 circuit. Proceedings of the National Academy of Sciences, 118(47). https://doi.org/10.1073/pnas.2110601118
[+] Other Work By Abigail O'Niel
Auditory Adderall: Auditory Beat Stimulation and Memory
Neuroanatomy
Modulation of neuronal activity in the auditory pathway using binaural 5 Hz beat firing differences improves recall memory, focus, and anxiety in a non-invasive manner.