The Synaptic Stability is Strong with This One
A recently published study shows that specific inhibitory cells have a strong and stable connection to cells associated with higher thought.
Author: David Stull
Neurophysiology
The brain likes to inhibit itself. While only 10% - 20% of cells in the brain are inhibitory, they play a crucial role in its proper functioning (Swanson and Maffei, 2019). When discussing how the brain functions, most talk is centered around excitatory electrical signals being sent throughout the brain via connected excitatory cells. While extensive research has been performed on connection strength between excitatory cells, there is a gap when considering connection strength of excitatory to inhibitory cells. In a 2021 study published in Cell Reports, Melander, et al. investigate the connection strength of excitatory to inhibitory cells by measuring the weight of the connection. They found that the excitatory to inhibitory connections weigh less and are more stable than excitatory to excitatory connections (Melander, et al., 2021).
A small amount of inhibition in the brain can provide a lot when considering complex behavior of neural systems (Thayer, 2006). Removal of inhibitory neurons from the hippocampal network can result in epileptic seizures and changes in behavioral patterns (Antonucci, et al., 2012). This shows that inhibitory neurons can act as a braking mechanism, regulating the rate of excitatory firing of other neurons. However, we know that the brain is plastic and will add, remove, and alter the strength of synapses over the course of time (Oberman and Pascual-Leone, 2013). Knowing whether an excitatory synapse is present can be observed by looking at the excitatory postsynaptic scaffolding protein, postsynaptic density-95 (PSD-95) (Keith and El-Husseini, 2008). PSD-95 has also been shown to play a role in synaptic strength and activity dependent plasticity (Beique and Andrade, 2003). Exciting inhibitory cells releases inhibitory neurotransmitter, acting as a regulator. Understanding the importance of the regulatory properties of exciting inhibitory cells, it would make sense for the synapse to be markedly stable.
Melander, et al. explored this by measuring the synaptic strength of excitatory layer 2/3 pyramidal neurons to parvalbumin+ (PV+) basket cells. These PV+ basket cells are inhibitory interneurons that can form synapses to other neurons or back to the stimulating cell. Measuring these synapses was performed by observing synaptic weight by measuring labeled PSD-95 abundance in each synapse. The synaptic weight assessment was performed for both pyramidal to PV+ synapses as well as pyramidal to pyramidal synapses. Then to assess the stability of the synapses, the survival rate was measured over 24 days and compared between the two synapse types (Melander, et al., 2021).
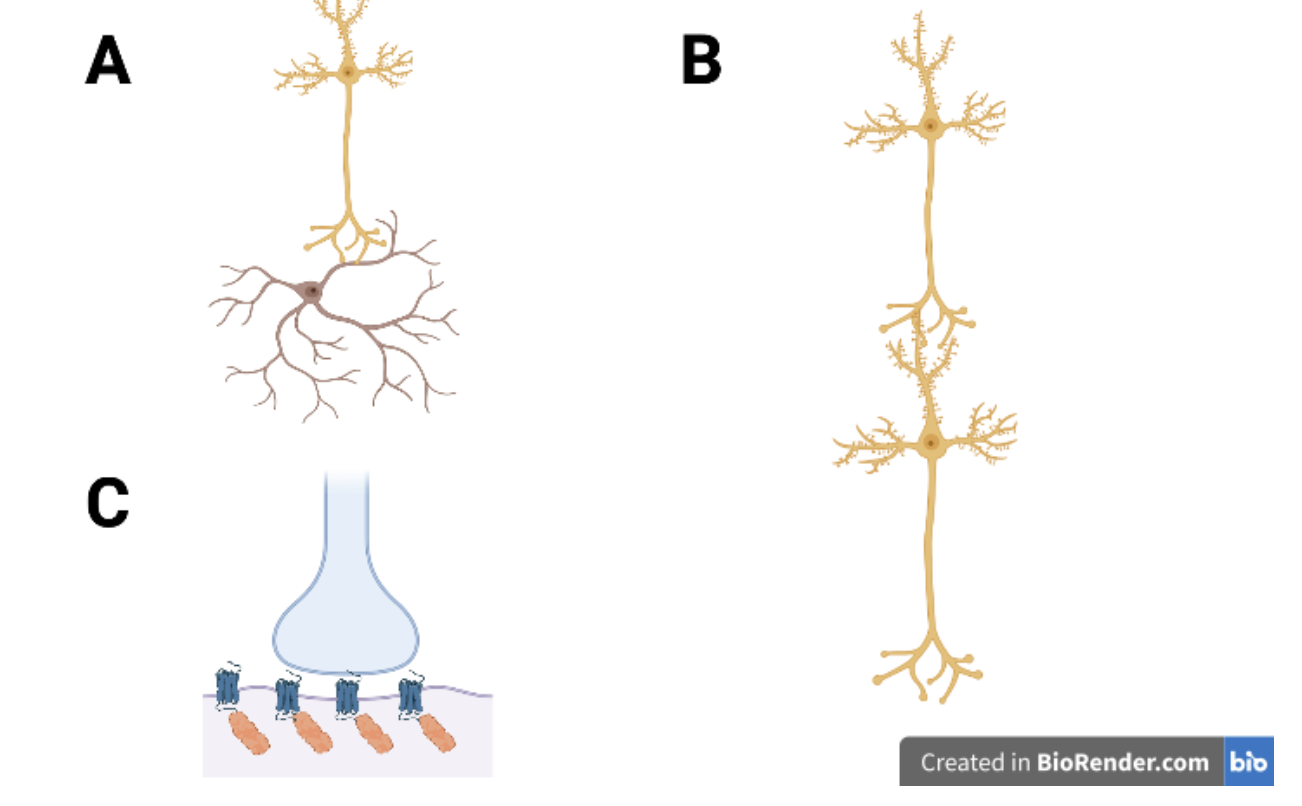
When looking at the synaptic weight between the pyramidal to pyramidal synapses and the pyramidal to PV+ synapses, the pyramidal to pyramidal synapse was more volatile than the pyramidal to PV+ synapse. This was observed by measuring the PSD-95 abundance over 24 days. On average, the pyramidal to pyramidal synapse had a larger change in synaptic weight and higher overall weight than the pyramidal to PV+ synapse (Melander, et al., 2021). Synaptic plasticity is the ability of neurons to strengthen or weaken their synapses and changes in PSD-95 abundance is associated with two forms of synaptic plasticity, long term potentiation (LTP) and long-term depression (LTD) (Tomita, Nicoll and Bredt, 2001). These changes would also be associated with a changing of the synaptic strength, rendering it more volatile or less reliable.
But when looking at the survival rate, the pyramidal to PV+ synapse had a much higher rate than the pyramidal to pyramidal synapse. This was observed at the 24 day mark when the pyramidal to PV+ synapse had a survival rate around 90% while the pyramidal to pyramidal synapse had a survival rate of around 75% (Melander, et al., 2021). Once again, this reiterates that the pyramidal to PV+ synapse is more stable than the pyramidal to pyramidal synapse.
Stability and proper function of synapses between inhibitory interneurons and excitatory cortical neurons is vital to maintain proper function of the brain. It was shown in a previous study that intermittent failure of inhibitory interneurons could allow for runaway excitation, resulting in seizures (Magloire, et al., 2018). It was also shown in another study that a decrease in frontal cortex PV+ cell density was associated with schizophrenia, suggesting that insufficient inhibition of cortical circuits propagated the condition (Kaar, et al., 2019). This again reinforces the importance of maintaining stable inhibition.
While a lot is known about synapses that involve pre and post synaptic excitatory cells, there is still a lot to be discovered with excitatory to inhibitory synapses as there is a wide variety of inhibitory cell types. The study by Melander, et al. provides a starting point to further explore other excitatory to inhibitory synapses like those observed in cholecystokinin+ basket cells and chandelier cells. The correlation between inhibitory cell stability and disease states like schizophrenia and epilepsy can provide another frame of reference to explore these conditions and target new therapeutics.
[+] References
Antonucci, F., Alpar, A., Kacza, J., Caleo, M., Verderio, C., Giani, A., Martens, H., Chaudhry, F. A., Allegra, M., Grosche, J., Michalski, D., Erck, C., Hoffmann, A., Harkany, T., Matteoli, M., & Hartig, W. (2012). Cracking down on inhibition: Selective removal of GABAergic interneurons from hippocampal networks. Journal of Neuroscience, 32(6), 1989–2001. https://doi.org/10.1523/jneurosci.2720-11.2012
Béïque, J. C., & Andrade, R. (2003). PSD‐95 regulates synaptic transmission and plasticity in rat cerebral cortex. The Journal of Physiology, 546(3), 859–867. https://doi.org/10.1113/jphysiol.2002.031369
Kaar, S. J., Angelescu, I., Marques, T. R., & Howes, O. D. (2019). Pre-frontal parvalbumin interneurons in schizophrenia: A meta-analysis of post-mortem studies. Journal of Neural Transmission, 126(12), 1637–1651. https://doi.org/10.1007/s00702-019-02080-2
Keith, D., & El-Husseini, A. (2008). Excitation control: Balancing PSD-95 function at the Synapse. Frontiers in Molecular Neuroscience, 1. https://doi.org/10.3389/neuro.02.004.2008
Magloire, V., Mercier, M. S., Kullmann, D. M., & Pavlov, I. (2018). GABAergic interneurons in seizures: Investigating causality with Optogenetics. The Neuroscientist, 25(4), 344–358. https://doi.org/10.1177/1073858418805002
Melander, J. B., Nayebi, A., Jongbloets, B. C., Fortin, D. A., Qin, M., Ganguli, S., Mao, T., & Zhong, H. (2021). Distinct in vivo dynamics of excitatory synapses onto cortical pyramidal neurons and parvalbumin-positive interneurons. Cell Reports, 37(6), 109972. https://doi.org/10.1016/j.celrep.2021.109972
Oberman, L., & Pascual-Leone, A. (2013). Changes in plasticity across the lifespan. Changing Brains - Applying Brain Plasticity to Advance and Recover Human Ability, 91–120. https://doi.org/10.1016/b978-0-444-63327-9.00016-3
Swanson, O. K., & Maffei, A. (2019). From hiring to firing: Activation of inhibitory neurons and their recruitment in behavior. Frontiers in Molecular Neuroscience, 12. https://doi.org/10.3389/fnmol.2019.00168
Thayer, J. F. (2006). On the importance of inhibition: Central and peripheral manifestations of nonlinear inhibitory processes in Neural Systems. Dose-Response, 4(1). https://doi.org/10.2203/dose-response.004.01.002.thayer
Tomita, S., Nicoll, R. A., & Bredt, D. S. (2001). PDZ protein interactions regulating glutamate receptor function and plasticity. Journal of Cell Biology, 153(5). https://doi.org/10.1083/jcb.153.5.f19
[+] Other Work By David Stull
The Roles of ROS1 and NTRK Fusions in Glioblastoma Multiforme
Neuroscience In Review