Protect Your Brain from Stress
Study shows being stressed in childhood hijacks your brain and makes you even more stressed in adulthood.
Author: Diana Trang
Neurophysiology
Introduction
Depression. A ten-letter word that has a huge effect on many people’s lives. According to The World Health Organization, about 280 million people in the world suffer from depression (WHO). Depression, also known as major depressive disorder, is a very common and serious illness that negatively affects how you feel, think, and act (Kanter et al., 2008). This causes many people to suffer and function poorly in their everyday lives at work, school, home, etc. The exact cause of depression is complicated. Genetic, biological, environmental, and psychological factors can play a role in increasing a person’s chance of developing depression. One common risk factor for depression is Early life stress (ELS) (Tan et al., 2021). Early life stress is described as physical, sexual, and emotional abuse that is experienced by a developing child. This would include any adverse childhood experience (ACE) such as neglect and separation from a parent, growing up with a substance abuser in the home, etc. In one study, rats were maternally separated after birth for several hours for 2 weeks (Campbell et al., 2017). The maternally separated ELS rats saw a decrease in pressing a bar for a reward-sucrose solution. This concludes that ELS reduced motivation for rewards, showing an effect on the reward circuit. In another study, conducted by the Center for Disease Control and Prevention showed a strong relationship with child adversities and general mental health problems in adulthood (Edwards et al., 2003). Recently the CDC, along with Kaiser Permanente, funded an epidemiological study and found that more than 64% of adults that responded had experienced ACE and were more likely to develop psychiatric or medical disorders (Targum & Nemeroff, 2019). There are many other studies that show the negative relationship between early stress and the brain. However, the specific mechanism to which and how early life stress leads to negative outcomes are not well understood. In the study published in the journal Nature Neuroscience, researchers investigated how ELS affects transcription, the process in which cells are being replicated using DNA or RNA within the nucleus accumbens (NAc), in mice (Kronman et al., 2021). This part of the brain is responsible for mediating emotional and motivation processing, reward, and pleasure processing, playing a major role in the brain’s reward circuit (Hyde & Garcia-Rill, 2019). The NAc is composed mostly of GABAergic projection neurons called medium spiny neurons (Soares-Cunha et al., 2020). Medium spiny neurons are known to express either D1 dopamine receptors (Drd1) and D2 dopamine receptors (Drd2). These two dopamine receptors help mediate the response of stress. These dopamine receptors are known to play an important role in the role of stress because they are key players in the brain's reward system (Baik, 2020). The brain either sends a signal for reward or not to cope with the stress. From previous research, the researchers already identified the demethylation H3K79me2 as a critical regulator of ELS-induced transcriptional abnormalities in the adult NAc (Pena et al., 2019). H3K79me2 is the name of the mechanism that mediates the effects of ELS. One way of looking at demethylation is that it turns the gene “on”. Researchers found that ELS increases changes in the D2-type of medium spiny neurons within the NAc. This is then linked to the increased susceptibility to stress in adulthood.
Methods
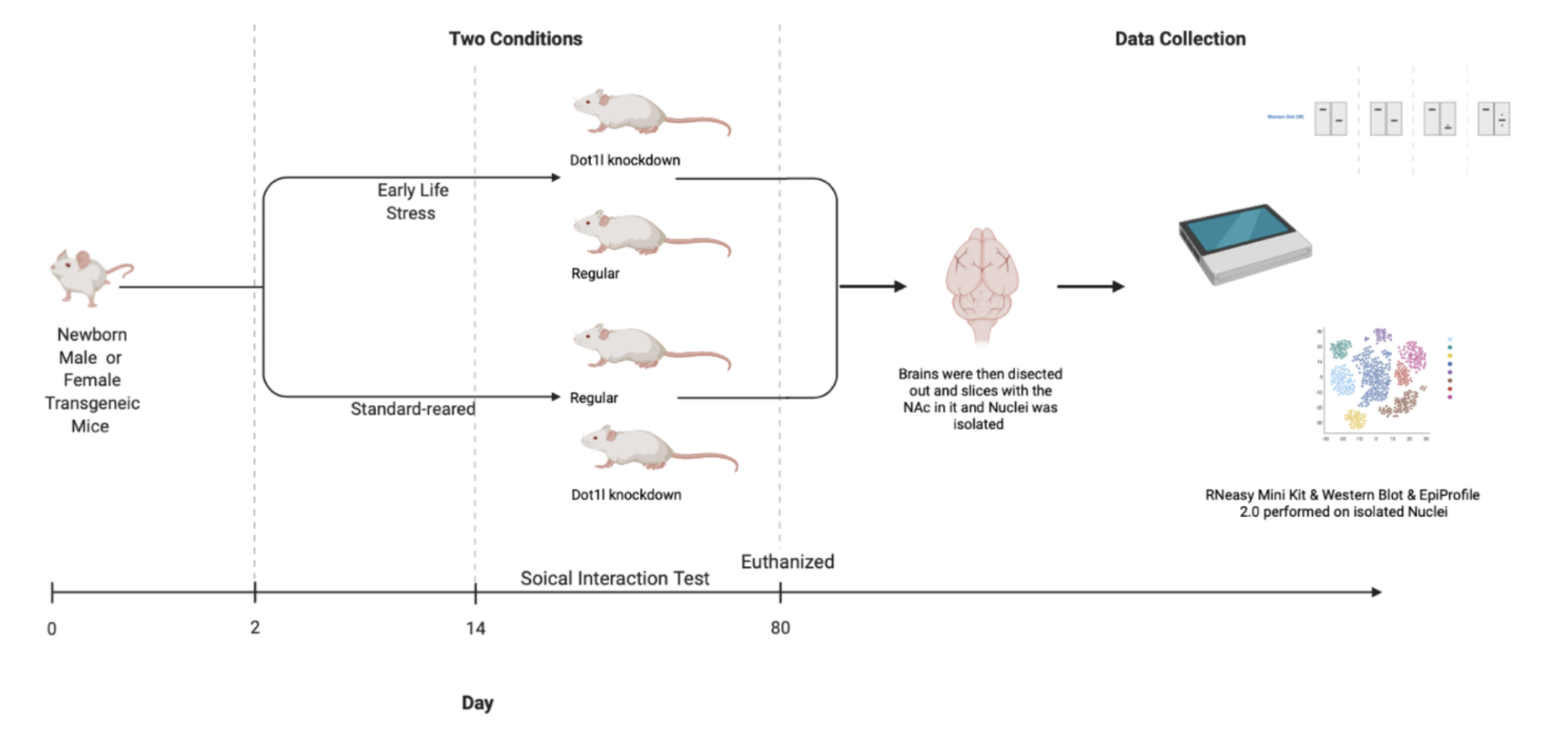
Researchers used transgenic male and female mice and were either Dot1l knockdown or not Knockdown mice basically means that they are mice that do not have a certain gene (Hall et al., 2009). In this case mice did or did not have the Dot1l enzyme. Knockdown mice are commonly used to suppress a certain gene for research. All mice were put in two different conditions. For the mice that would experience ELS, they were maternally separated for 4 hours a day after day 2 of birth and the standard-reared (Std) were not separated from their mothers after birth for 14 days. The social interaction of the mice was also observed by the researchers after the 14 days. The mice were kept for 80 days (adulthood). During that time, the mice were placed in different social interaction tests, such as a forced swim immobility test and an open field exploration test. The mice were then euthanized and their brains were then dissected out once they reached adulthood. The results were then recorded. Afterwards, thin slices of the brain containing the NAc were collected and frozen for use. Once some of the slices were ready to use, the nuclei were then isolated from the frozen tissue in order to isolate the D2 nuclei. Histone proteins were then collected from the nuclei with acid overnight. After the proteins dried, the histone peptide solution was collected and placed into a nano-flow liquid chromatograph and then a mass spectrometer. Once the data was all collected, it was then analyzed using an EpiProfile 2.0. This helped extract the ions chromatograms for (un)modified histone peptides. In addition, RNA was also extracted from the whole tissue using RNeasy Mini Kit and was sequenced. This was done by taking slices of the frozen brain. A western blot was then performed to detect the different proteins. Protein expression was then analyzed. Once all the data was collected, different statistical calculations were performed. Researchers also performed protein isolation with the help of a western blot. Reference Figure 1 for the visual of the methods.
Results
RNA sequencing showed that the most regulated enzyme from ELS was Dot1l, the enzyme for H3K79 methylation, which signifies Dotl1 helped catalyze H3K79me2. This means that this enzyme is what regulates the genetic material being activated. It was seen that Dot1l was enriched in the D2 medium spiny neurons for both male and female mice. With the ELS mice, it was seen that there was an increased expression of Dot1l. This was then seen to be magnified in adulthood.
When the Dot1l had an overexpression in the NAc of the D2 medium spiny neurons of the Std mice saw the same behavior of ELS-induced behavior phenotypes, showing impaired social interaction. Increasing the Dot1l in the medium spiny neurons saw that there was an increase in stress vulnerability. This meant that ELS would cause the mice to be more stressed out or more susceptible to stress later in life. When the Dot1l was decreased, it decreased stress vulnerability. This means that the mice were likely to be less stressed out and less likely to be susceptible to stress.
Conclusion
With the help of RNA sequencing, researchers were able to determine that the overexpression of Dot1l enhances the gene expression changes that are supposed to occur from early life stress. However, they saw that with the Dot1l knockdown, the gene expression changes were not able to happen with early life stress. This means that this mechanism can play a role in reprogramming the D2 medium spiny neurons in the NAc, leading to the levels of stress susceptibility to change later in life. Based on the results of the study, it can be seen that the Dot1l was present, which can change the H3K79me2 mechanism. This means that future research can be conducted to create a pharmaceutical that can target the specific gene expression to help control stress-induced susceptibility. It possibly means that if this is early life stress is preventable, it could prevent later stress vulnerability. This may be done by using a small molecule inhibitor of Dot1l. By inhibiting the enzyme, it would decrease the stress vulnerability in adulthood. This could mean that in the future a pharmaceutical could be used to target to help change the mechanisms and reprogram the cell. This study also further shows that ELS can play a role in how stress is perceived in adulthood.
[+] References
Baik, JH. Stress and the dopaminergic reward system. Exp Mol Med 52, 1879–1890 (2020). https://doi.org/10.1038/s12276-020-00532-4
Campbell et al. (2017). Chemogenetic activation of the lateral hypothalamus reverses early life stress-induced deficits in motivational drive. European Journal of Neuroscience, 46(7): 2285-2296.
Edwards et al. (2003). Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am. J. Psychiatry, 160 (2003), pp. 1453-1460
Hall et al. (2009). Overview: generation of gene knockout mice. Current protocols in cell biology, Chapter 19, Unit–19.12.17. https://doi.org/10.1002/0471143030.cb1912s44
Hyde, J & Garcia-Rill E, Chapter 6 - Autism and arousal, Editor(s): Edgar Garcia-Rill, Arousal in Neurological and Psychiatric Diseases, Academic Press, 2019, Pages 83-114, ISBN 9780128179925. https://doi.org/10.1016/B978-0-12-817992-5.00006-4.
Kanter et al. (2008). The nature of clinical depression: symptoms, syndromes, and behavior analysis. The Behavior analyst, 31(1), 1–21. https://doi.org/10.1007/BF03392158
Kronman et al. (2021) Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat Neurosci 24, 667–676 (2021). https://doi.org/10.1038/s41593-021-00814-8
Tan et al. (2021). Influence of early life stress on depression: from the perspective of neuroendocrine to the participation of gut microbiota. Impact Journal on Aging. 13(23): 25588-25601.
Targum, S. D., & Nemeroff, C. B. (2019). The Effect of Early Life Stress on Adult Psychiatric Disorders. Innovations in clinical neuroscience, 16(1-2), 35–37.
Pena et al. (2019). Early life stress alters transcriptomic patterning across reward circuitry in make and female mice. Nat. Comm. 10, 5089.
Soares-Cunha et al. (2020). Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Molecular psychiatry, 25(12), 3241–3255. https://doi.org/10.1038/s41380-019-0484-3
World Health Organization. (n.d.). Depression. World Health Organization. Retrieved April 26, 2022, from https://www.who.int/news-room/fact-sheets/detail/depression