Therapeutic Potential to Treat Neuropsychiatric and Neurodevelopment Disorders (NDDs)
A 2019 study focuses on cortical circuits to help researchers better examine the therapeutic potential to treat neuropsychiatric and neurodevelopmental disorders (NDDs) in drug-resistant epilepsy patients.
Author: Mai Suong Vo
Neurophysiology
Introduction
The CDC estimates that 1 in 6 children or about 17% of children ages 3 to 17 years old in the U.S live with one or more developmental disabilities (CDC, 2019). From 2009-2011 to 2015-2017 there was a significant increase (16.2%, P<.001) in the prevalence of developmentally disabled, ADHD, and autism (Zablotsky et al., 2019). Neuropsychiatric and neurodevelopmental disorders, known as NDDs are a class of disorders that affect brain development and function that involve wide genetic and clinical variabilities. NDDs comprise intellectual disability (ID), Communication Disorders, Autism Spectrum Disorder (ASD), Attention-Deficit/Hyperactivity Disorder (ADHD), and many more (Parenti et al, 2020). In studies, it is known that NDDs do not have a cure but do have current hands-on treatment approaches. Such as psychical therapy, occupational therapist, and speech-language pathologist (Kennedy Krieger Institute). Therefore, further research can be focused on the therapeutic potential of neuropsychiatric and neurodevelopmental disorders.
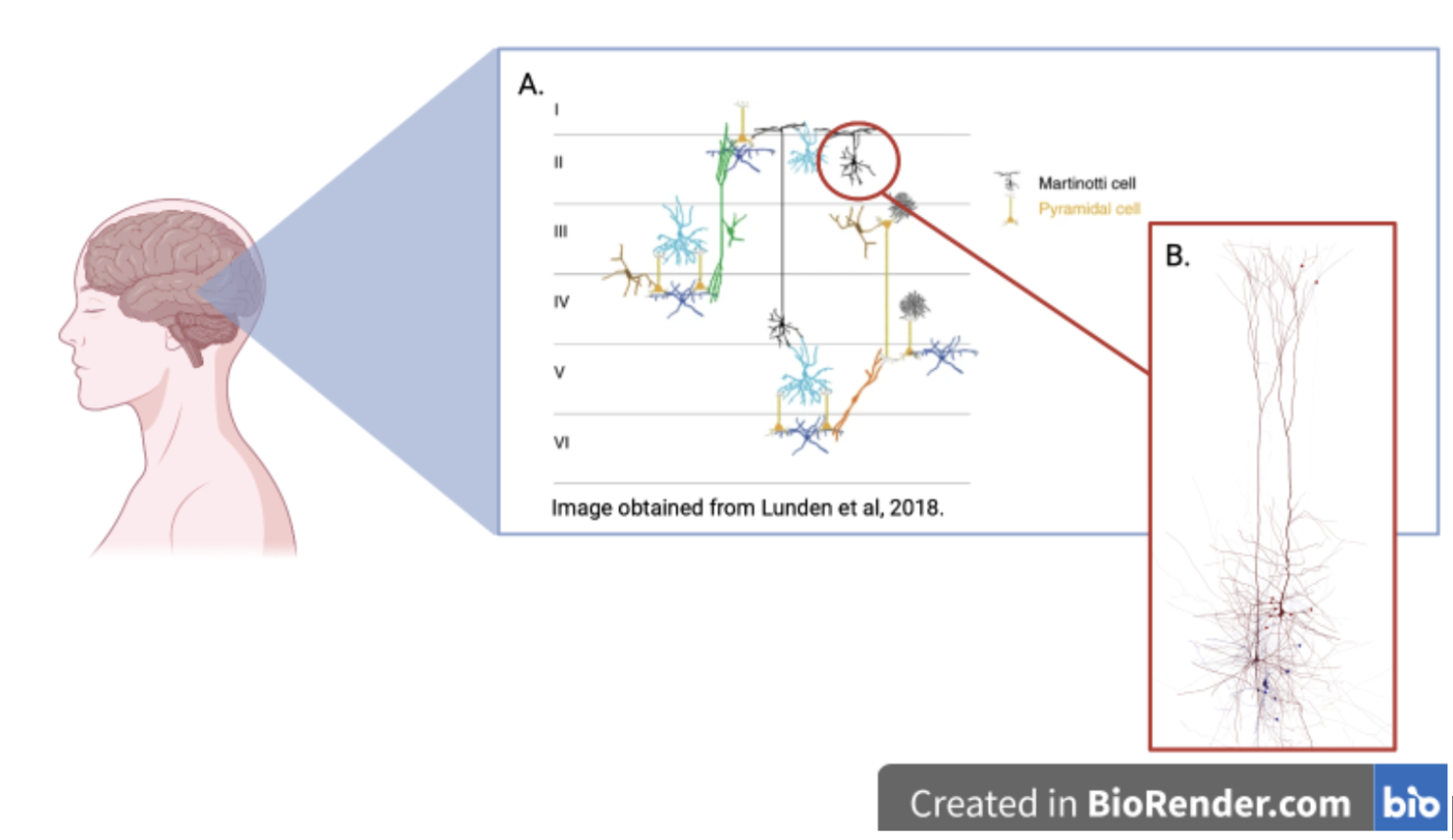
A study done in 2019 published by Frontiers in Cellular Neuroscience investigated possible potential therapeutic targets by looking at a specific receptor named Group I metabotropic glutamate receptor (mGluRs) (Kroon et al., 2019). This receptor was addressed in the human neocortex. This study found that activation of the receptor (mGluRs) in Martinotti cells lead to an increased action potential. Martinotti cells, known as MS, are small multipolar neurons with short branching dendrites (Figure 1). This study will help fill in research gaps involving specific targeting mechanisms, to help better support the NDDs community.
Background
To understand potential treatments for NDDs in participants that suffer from drug-resistant epilepsy, we must discuss what happens in the brain when it occurs. Epilepsy is known as a seizure disorder where the brain is disturbed (Thijs et al., 2019). Patients that have reoccurring (2+) seizures are diagnosed with epilepsy. This is a central nervous system (CNS) disorder affecting both males and females, of all ages, and ethnic backgrounds. While many seizure explanations may look different, there are similarities in mechanism formation. Seizures occur when they are excitation of a group of nerves and have too little inhibition (Mandal, 2019). This can be caused by inward currents like sodium, and calcium or excitatory neurotransmitters like glutamate (Gerfern et al., 2018). Another key understanding would be the cortical circuit. Cortical circuits are categorized into two main neuron classes, excitatory and inhibitory (Wolf et al., 2014). One of the main differences between the two classes is that excitatory neurotransmitters increase action potential (AP) firing whereas inhibitory neurotransmitters decrease the likelihood that the neuron will fire an action. In the human cortex, there are six layers of the cortical cortex (cortical layer 1-V1).
Methodology
In this study conducted by Kroon and colleagues, twenty females and twenty males (n=40) were evaluated. The age range was seventeen (17) to sixty-three (63) years old. Each participant suffered from drug-resistant epilepsy. Some diagnoses consisted of medial temporal sclerosis (MTS), Tumor, Dysplasia, etc. First, cortical samples from the 40 participants were obtained through surgery. To observe depolarization or activation of Martinotti Cells (MC), many tests were run in one experiment and evaluated closely. Current-clamp recordings of the MC layer for pre-application and during an agonist application named DHPG ((S)-3,4-Dihydroxyphenlyhlycine) were analyzed. For further evaluation, double-labeling immunohistochemistry was done.
Results
This study found that receptor (mGluR) activation increases the inhibition of human pyramidal neurons. Another finding was that mGluR strongly activated MS in the human cortex. This was done by performing current-clamp recording onto MS in layer 2/3 to see if the receptor activation would change in membrane potential. Using the double-labeling immunohistochemistry, researchers confirmed that the agonist DHPG mediated its effect directly via MS.
Conclusion
The objective of this study was to investigate the activation of Martinotti cells in the cortical circuitry. Findings from this study have shown that the receptor mGluRs strongly activates MS in the human cortex, leading to even more activation of its neighboring neurons. When an inhibitory (-) and excitatory (+) neuron fires at the same time, there would not be a significant increase in action potentials. Pyramidal neurons are known to communicate via bursts of action potentials (Williams et al., 1999). This study proposed that when an agonist named DHPG is used in MC, it would inhibit it from bursting. Most importantly, this study identifies the depolarization of MS as a potential mechanism in NDDs. Previous research has shown that the activation of mGluRs in the hippocampus caused an increase in epileptogenesis activity (Merland et al., 1997). Epileptogenesis is simply the process during which changes occur in the brain after an injury. Another study was done in 2006 supporting this research proposing that the receptor mGluR is greatly involved in epilepsy (McNamara et al., 2006). With a growing interest in the mGluR-mediated neuronal transformation to develop recurrent seizures, this study provided good input. Though the findings of Kroon et al.’s 2019 study were significant, future research can focus on using these data to formulate testable treatments for patients suffering from these disorders. While the research itself was neurologically complex, more physiological explanations could have been done to help readers grasp the connection between the results and the therapeutic potential goal. In conclusion, this study was effective in presenting a small result within a larger picture in hopes that this can open doors for future MC and NDDs research.
[+] References
Gerfen, C. R., Economo, M. N., & Chandrashekar, J. (2018). Long distance projections of cortical pyramidal neurons. Journal of neuroscience research, 96(9), 1467-1475.
Kroon, T., Dawitz, J., Kramvis, I., Anink, J., Obermayer, J., Verhoog, M. B., ... & Meredith, R. M. (2019). Group I mGluR-mediated activation of martinotti cells inhibits local cortical circuitry in human cortex. Frontiers in Cellular Neuroscience, 13, 315.
Lee, C. K., & Huguenard, J. R. (2011). Martinotti cells: community organizers. Neuron, 69(6), 1042-1045.
Lunden, J.W., Durens, M., Phillips, A.W. et al. Cortical interneuron function in autism spectrum condition. Pediatr Res 85, 146–154 (2019).
Mandal, A. (2019). Epilepsy Pathophysiology. News Medical Life Science.
McNamara J. O., Huang Y. Z., Leonard A. S. (2006). Molecular signaling mechanisms underlying epileptogenesis. Sci. Signal.2006:re12. 10.1126/stke.3562006re12
Merlin L. R., Wong R. K. S. (1997). Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J. Neurophysiol. 78 539–544.
Parenti I, Rabaneda LG, Schoen H, Novarino G. (2020). Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. (8):608-621. doi: 10.1016/j.tins.2020.05.004. Epub 2020 Jun 5. PMID: 32507511.
Thijs, R., Surges, R., O’Biren., T., Sander, J. (2019). Epilepsy in adults. National Library of Medicine, Elsevier. DOI: 10.1016/S0140-6736(18)32596-0
Wang, Y., Toledo‐Rodriguez, M., Gupta, A., Wu, C., Silberberg, G., Luo, J., & Markram, H. (2004). Anatomical, physiological, and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. The Journal of physiology, 561(1), 65-90.
Wolf, F., Engelken, R., Puelma-Touzel, M., Weidinger, J. D. F., & Neef, A. (2014). Dynamical models of cortical circuits. Current opinion in neurobiology, 25, 228-236.
Williams, S. R., & Stuart, G. J. (1999). Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. The Journal of physiology, 521(Pt 2), 467.
Zablotsky B, Black LI, Maenner MJ, Schieve LA, Danielson ML, Bitsko RH, Blumberg SJ, Kogan MD, Boyle CA. (2019) Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009-2017. Pediatrics.144(4):e20190811. doi: Zablotsky B, et al.