Amyloid-β induces pyroptotic neuronal death in Alzheimer’s Disease
Author: Alex Jensen
Download: [ PDF ]
Neuroscience In Review
Introduction
The most common cause of dementia in adults is Alzheimer’s Disease (AD), with approximately 6.2 million Americans aged 65 or older living with AD in 2021 (Alzheimer’s Association, 2021). AD is a neurodegenerative disease characterized by impaired memory and gradually declining cognitive ability. This is thought to be caused by the building up of amyloid-β (Aβ) protein which leads to neuronal death, progressively worsening the individual’s cognitive impairment. Due to the visible burden of Aβ plaques in the brain of an AD patient, most research and treatment has been focused on clearing these plaques to improve cognitive decline. Instead, I propose putting more effort into identifying the mechanism of neuronal death triggered by Aβ protein in AD and inhibiting neuronal death to improve cognition. Recent research has led to pyroptosis as a novel target for cell death in AD. Pyroptosis is an inflammatory cell death pathway that is regulated by inflammasome and caspase activation, resulting in cell lysis. This is a normal, regulated cell death pathway used to help the body fight infection, but the pathway may be overactivated in the presence of Aβ in AD. Experiments have shown increased inflammatory factors, pyroptotic proteins, and cell lysis in response to Aβ, supporting pyroptosis as the cause of neuronal death in AD. Specifically the GSDMD protein, an essential protein for pyroptotic cell lysis, showed such an elevated expression in AD patients that it has been investigated as a biomarker for AD, indicating the primary use of pyroptosis (Shen et al, 2021). If pyroptosis is the cause of neuronal death in AD, and subsequent cognitive decline, inhibiting pyroptosis has the potential to improve cognitive impairment in AD. Preliminary research has shown improved cognitive function and reduced inflammatory response in AD mice when pyroptosis is inhibited (Li et al, 2021 & Li et al, 2020). This indicates a strong potential for treating AD with pyroptosis inhibition, though further clinical research on inhibition of pyroptosis is needed.
The pyroptotic cell death pathway
Pyroptosis is characterized by pore formation in the plasma membrane which allows proinflammatory factors to be released. Pores continue to form and the cell swells before finally rupturing. The pyroptosis pathway begins with inflammasome activation which can be triggered by pattern recognition receptors (PRRs) in response to danger-associated molecular patterns (DAMPs) in the extracellular space (Walle & Lamkanfi, 2016). These DAMP receptors respond to endogenous molecules released by damaged cells and activate an immune response (Gong et al, 2020). Activated inflammasomes then recruit caspase-1 which cleaves GSDMD and proIL-1β and proIL-18, resulting in GSDMD-N, IL-1β, and IL-18. GSDMD-N is used to form the transmembrane pores in pyroptosis (Kuang et al, 2017). The pores formed by GSDMD-N allow the proinflammatory cytokines IL-1β and IL-18 to be released. IL-1β and IL-18 are also monitored by DAMP receptors and therefore exacerbate the pyroptotic inflammatory response. Aβ has previously been implicated as a DAMP, recognized by DAMP receptors and therefore a catalyst to pyroptosis, elucidating pyroptosis as a mechanism of neuronal death in AD (Clark & Vissel, 2015).
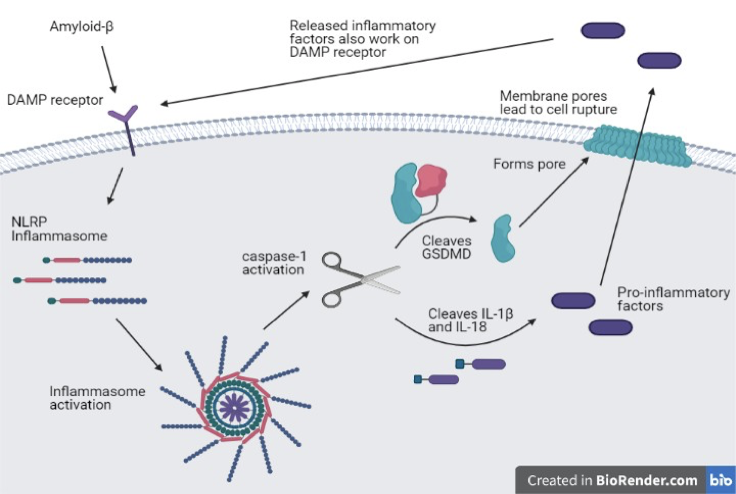
Inflammasome activation, GSDMD, and IL-1β in AD
GSDMD-N is required for pore formation in pyroptosis, therefore increased expression of GSDMD-N is highly indicative of increased pyroptosis activation (Shi et al, 2015). AD patients have heavily increased GSDMD expression compared to healthy individuals. Elevated expression levels of this pyroptotic precursor in cerebrospinal fluid were found to have a 99.98% accuracy in diagnosing AD in humans, demonstrating the predominant use of pyroptosis in AD (Shen et al, 2021).
To further illuminate the neuronal death mechanism associated with Aβ, AD mouse models have been examined with respect to pyroptotic factors. Consistent upregulation of NLRP3 and NLRP1 inflammasomes and increased expression of caspase-1 in AD mice indicates inflammasome activation in AD (Heneka et al, 2013 and Tan et al, 2014). The increased Aβ protein in AD causes the activation of these inflammasomes through DAMP receptors and begins the inflammatory response, allowing caspase-1 to cleave GSDMD and execute pyroptosis. Upregulation of the inflammasomes in addition to increased Aβ deposition results in overactivation of this pyroptotic pathway. An increase in cleaved GSDMD and IL-1β with increased neuronal death suggests the use of pyroptosis in response to Aβ in mouse cortical neurons (Han et al, 2020).
Inhibiting pyroptosis in AD
Inhibiting pyroptosis via caspase-1 or NLRP3 inflammasome inhibition have both been shown to reduce the Aβ plaque burden in AD mice brains, supporting pyroptosis inhibition as a potential treatment for AD (Flores et al, 2018 and Dempsey et al, 2017). Inhibiting the NLRP3 inflammasome has been effective in improving cognition and reducing neuroinflammation in AD mice. After using an NLRP3 inflammasome inhibitor, AD mice showed a significant improvement in spatial memory, but did not completely return to levels of the control mice (Li et al, 2020).
Similarly, inhibiting the NLRP1 inflammasome in AD mice also improves cognition and neuroinflammation. Though neuroinflammation and spatial memory were improved after NLRP1 inhibition, the mice were still at a deficit compared to healthy mice (Li et al, 2021). Inhibition of both the NLRP3 and NLRP1 inflammasomes simultaneously may be more effective in improving cognition and neuroinflammation. Inhibiting both inflammasomes would further reduce levels of pyroptosis in the AD mice, therefore reducing AD symptoms associated with the inflammatory cell death pathway. Caspase-1 inhibition also proves effective in reducing neuroinflammation and improving cognitive function in AD mice. After caspase-1 inhibition, AD mice exhibit improved cognition and reduced neuroinflammation but again did not return to levels of healthy mice (Flores et al, 2020). This suggests there are additional aspects to cognitive decline in AD aside from pyroptosis, though pyroptosis does play a significant role in the neuronal loss in AD.
Conclusions and future directions
Inhibiting aspects of the pyroptotic pathway improves cognition and neuroinflammation in AD mice, indicating that pyroptosis is overactivated in AD. Inhibition of caspase-1 can effectively reduce neuronal loss in AD by functionally blocking GSDMD from being cleaved, preventing the formation of the transmembrane pores necessary for pyroptosis. Additionally, the inhibition of inflammasomes reduced pyroptosis activation, but there are multiple inflammasomes that can recruit caspase-1 and therefore multiple inflammasomes would need to be inhibited to produce the same effect as inhibiting only caspase-1. A next step in research includes inhibiting activation of both NLRP3 and NLRP1 at the same time, this may produce synergistic effects in reducing neuroinflammation in AD mice compared to inhibiting only one inflammasome. Depending on the severity of pyroptosis activation, different methods of inhibition can also be used but further research is needed on inhibiting multiple forms of pyroptosis simultaneously in animal models. For cases with more severe neuroinflammation pyroptosis may be more heavily activated, therefore multiple pyroptotic pathways may need to be inhibited to effectively reduce the inflammation and improve cognition.
Regulating pyroptosis has been used for treatment of different diseases, including inflammatory diseases and cancers, but has yet to be utilized as a treatment for neuroinflammation in humans (Zheng & Li, 2020). Disulfiram is an example of an FDA-approved drug that inhibits pyroptosis, blocking GSDMD from forming membrane pores and preventing the release of proinflammatory cytokines (Hu et al, 2020). A second example of a pyroptosis inhibiting FDA-approved drug is thalidomide. Thalidomide is used for treating inflammatory skin diseases by inhibiting caspase-1 activation and thereby inhibiting pyroptosis (Yu et al, 2021 and Keller et al, 2009). In addition, there are a variety of FDA-approved NLRP3 inflammasome inhibitors used to suppress immune response in different disorders (Zahid et al, 2019). Since there is evidence of pyroptosis inhibition successfully reducing inflammation elsewhere in the body, there is a strong likelihood pyroptosis inhibition can be used to reduce neuroinflammation in AD and therefore improve cognition. Since animal testing for pyroptosis inhibition in AD has been successful, the next step would be volunteer clinical trials. Medications usually go through testing on healthy individuals to find correct dosage and ensure no unexpected side effects before being used on individuals with the disorder (Alzheimer’s Research UK). Current use of pyroptosis inhibition in humans provides a foundation for the correct dosage and effect in humans, thereby accelerating the path to clinical trials for pyroptosis inhibition in AD patients.
[+] References
Alzheimer’s Association. (2021). Alzheimer’s Disease Facts and Figures. Alzheimer’s Dementia 17(3): 19.
Alzheimer’s Research UK. Clinical Trials.
Clark, I.A., Vissel, B. (2015). Amyloid β: one of there danger-associated molecules that are secondary inducers of the proinflammatory cytokines that mediate Alzheimer’s disease. British Journal of Pharmacology 172(15): 3714-3727.
Dempsey, C., Araiz, A.R., Bryson, K.J., Finucane, O., Larkin, C., Mills, E.L., Robertson, A.A.B., Cooper, M.A., O’Neill, L.A.J., Lynch, M.A. (2017). Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain, Behavior, and Immunity 61: 306-316.
Flores, J., Noel, A., Foveau, B., Beauchet, O., LeBlanc, A.C. (2020). Pre-synaptic Caspase-1 inhibitor delays cognitive decline in a mouse model of Alzheimer disease and aging. Nature Communications 11(4571): 1-14.
Flores, J., Noel, A., Foveau, B., Lynham, J., Lecrux, C., LeBlanc, A.C. (2018). Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nature Communications 9(3916): DOI: 10.1038/s41467-018-06449-x.
Gong, T., Liu, L., Jiang, W., Zhou, R. (2020). DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nature Reviews Immunology 20: 95-112.
Han, C., Yang, Y., Guan, Q., Zhang, X., Shen, H., Sheng, Y., Wang, J., Zhou, X., Li, W., Guo, L., Jiao, Q. (2020). New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. Journal of Cellular and Molecular Medicine 24(14): 8078-8090.
Heneka, M.T., Kummer, M.P., Stutz, A., Delekate, A., Schwartz, S., Saecker, A., Griep, A., Axt, D., Remus, A., Tzeng, T., Gelpi, E., Halle, A., Korte, M., Latz, E., Golenbock, D. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434): 674-678.
Hu, J.J., Liu, X., Xia, S., Zhang, Z., Zhang, Y., Zhao, J., Ruan, J., Luo, X., Luo, X., Bai, Y., Wang, J., Hollingsworth, R., Magupalli, V.G., Zhao, L., Luo, H.R., Kim, J., Lieberman, J., Wu, H. (2020). FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nature Immunology 21: 736-745.
Keller, M., Sollberger, G., Beer, H.D. (2009). Thalidomide Inhibits Activation of Caspase-1. Journal of Immunology 183(9): 5593-5599.
Kuang, S., Zheng, J., Yang, H., Li, S., Duan, S., Shen, Y., Ji, C., Gan, J., Xu, X., Li, J. (2017). Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. PNAS 114(40): 10642-10647.
Li, J., Zhuang, L., Luo, X., Liang, J., Sun, E., He, Y. (2020). Protection of MCC950 against Alzheimer’s disease via inhibiting neuronal pyroptosis in SAMP8 mice. Experimental Brain Research 238: 2603-2614.
Li, Q., Wang, Q., Guan, H., Zhou, Y., Liu, L. (2021). Schisandrin inhibits NLRP1 inflammasome- mediated neuronal pyroptosis in mouse models of Alzheimer’s disease. Neuropsychiatric Disease and Treatment 17: 261-268.
Shen, H., Han, C., Yang, Y., Guo, L., Sheng, Y., Wang, J., Li, W., Zhai, L., Wang, G., Guan, Q. (2021). Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain and Behavior. DOI: 10.1002/brb3.2063.
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., Zhuang, Y., Cai, T., Wang, F., Shao, F. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660-676.
Tan, M.S., Tan, L., Jiang, T., Zhu, X.C., Wang, H.F., Jia, C.D., Yu, J.T. (2014). Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death and Disease 5(8): e1382.
Walle, L.V., Lamkanfi, M. (2016). Pyroptosis. Current Biology 26(13): R568-R572.
Yu, P., Zhang, X., Liu, N., Tang, L., Peng, C., Chen, X. (2021). Pyroptosis: mechanisms and diseases. Signal Transduction and Targeted Therapy 6(128): DOI: 10.1038/s41392-021-00507-5.
Zahid, A., Li, B., Kombe, A.J.K., Jin, T., Tao, J. (2019). Pharmacological Inhibitors of the NLRP3 Inflammasome. Frontiers in Immunology 10(2538): DOI: 10.3389/fimmu.2019.02538.
Zheng, Z., Li, G. (2020). Mechanisms and Therapeutic Regulation of Pyroptosis in Inflammatory Diseases and Cancer. International Journal of Molecular Sciences 21(4): 1456.
[+] Other Work By Alex Jensen
Predicting nausea using AI
Neuroanatomy
A new study shows that artificial intelligence may be able to predict an individual's susceptibility to nausea.
Impact of Air Pollution on the Brain
Neurophysiology
Exposure to diesel exhaust particles causes neuroinflammation, impaired memory, and anxious behaviour in mice.