Salt on the brain: a possible explanation for the irresistibility of salty foods
A new study combines several genetic tools to identify a neural circuit in mice that synthesizes information about taste and internal state to modulate appetite for sodium. Similar techniques could be used to probe a number of other peripheral circuits.
Author: Jacob Pennington
Download: [ PDF ]
Neurophysiology
Mouth-watering cravings for salty foods like french fries or mozzarella sticks are something most people can probably identify with, but the source of these cravings in the brain has long remained unclear1. Several studies have identified brain regions that are associated with appetite for sodium – one kind of important salt – but what exactly those regions do is not known. In a study recently published in Nature, Lee et al. sought to investigate the role of one such brain region by switching its activity in mice on or off while monitoring sodium appetite. The researchers found that stimulation of this region tended to increase appetite for sodium and that consuming sodium subsequently decreased the region’s activity but only if the mice were able to taste the sodium. The comprehensive strategy used in this study could be applied to other sensory modalities and internal drives to solidly advance our understanding of how the brain responds to daily needs.
BACKGROUND
Neurons in the pre-locus coeruleus (pre-LC) express c-FOS, a marker for increased activity, in response to a prolonged low-sodium diet.2 Relatedly, a population of HSD2-positive neurons in the nucleus of the solitary tract (NTS) drives appetite for sodium when stimulated, and this region projects to the aforementioned pre-LC neurons.3 Both of these regions are therefore likely involved in balancing sodium concentrations in the body by driving sodium appetite, a “hard-wired” drive akin to thirst that can impact blood pressure and other health metrics.4,5,6 The study by Lee et al. focuses on the pre-LC region, but also demonstrates vital forward projections from the NTS to the pre-LC region (results omitted for brevity).
METHODS
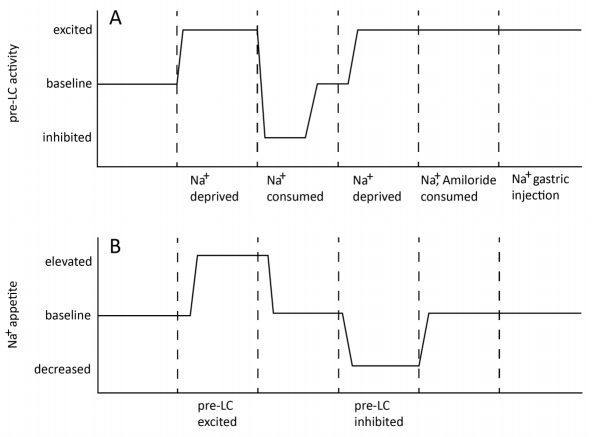
To assess the causes and results of pre-LC activation, the authors first measured c-FOS expression in the pre-LC under sodium-depleted, water-depleted, sodium-rescued, and control conditions.7 Then the authors used an adeno-associated viral (AAV) delivery system to cause pre-LC neurons to be responsive to light stimulation via Cre-dependent channelrhodopsin.8,9 Two variations on this procedure were used to allow either optogenetic excitation or inhibition of the pre-LC, and the authors observed the effects of both types of stimulation on sodium appetite and behavior. The authors also monitored pre-LC activity, measured via calcium fluorescence due to GCaMP6s expression, before and after sodium intake. This measurement was repeated with the addition of amiloride to block taste sensation of sodium.10,11 The authors also measured activity before and after injection of sodium directly into the mice’s stomachs.
RESULTS
A significant number of pre-LC neurons expressed c-FOS in the sodium-depleted condition but not in other conditions, indicating that the neurons became active in response to a lack of dietary sodium. Optogenetic excitation of the pre-LC resulted in increased sodium consumption, aversion to stimulation in a place preference task, and increased effort to avoid stimulation in a leverpressing task. The researchers interpreted this to mean that pre-LC activity both induced appetite for sodium and was perceived as unpleasant. Optogenetic inhibition of pre-LC activity instead resulted in decreased sodium consumption. Ingestion of sodium by deprived mice quickly suppressed pre-LC activity under normal conditions, but not when the mice were also treated with amiloride. Gastric injection of sodium did not affect pre-LC activity. These results indicated that sodium’s impact on pre-LC activity was directly tied to the sensation of tasting the sodium.
CONCLUSION
The results presented by Lee et al. clearly demonstrate a causal interaction between sodium taste and pre-LC neural activity, solidifying the region’s role as a taste-mediated regulator of sodium appetite. Further, the authors found that peripheral impacts on pre-LC neurons were eliminated if the NTS was inhibited, placing these results (summarized in Figure 1) in the context of a welldefined circuit. The fact that pre-LC activity, and sodium appetite in turn, is significantly modulated by sodium taste (but not consumption) has interesting implications for the moderation of sodium intake in humans. Of more general significance from this study is its demonstration of how calcium fluorescence, optogenetics, behavioral measures, and genetic markers can be combined to directly probe the function of neural circuits.
[+] References
Lee, S., Augustine, V., Zhao, Y., Ebisu, H., Ho, B., Kong, D., and Oka, Y. (2019). Chemosensory modulation of neural circuits for sodium appetite. Nature, 568: 93-97. doi: 10.1038/s41586-019-1053-2
Geerling, J., Stein, M., Miller, R., Shin, J., Gray, P., and Loewy, A. (2011). FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Research, 1375: 19-27. doi: 10.1016/j.brainres.2010.11.028
Jarvie, B. and Palmiter, R. (2017). HSD2 neurons in the hindbrain drive sodium appetite. Nature Neuroscience, 20(2): 167-169. doi: 10.1038/nn.4451
Farquhar, W., Edwards, D., Jurkovitz, C., and Weintraub, W. (2015). Dietary Sodium and Health: More Than Just Blood Pressure. Journal of the American College of Cardiology, 65(10): 1042-1050. doi: 10.1016/j.jacc.2014.12.039
Geerling, J. and Loewy, A. (2008). Central regulation of sodium appetite. Experimental Physiology, 93(2): 177-209. doi: 10.1113/expphysiol.2007.039891
Johnson, A. and Thunhorst, R. (1997). The Neuroendocrinology of Thirst and Salt Appetite: Visceral Sensory Signals and Mechanisms of Central Integration. Frontiers in Neuroendocrinology, 18: 292-353. doi: 10.1006/frne.1997.0153
Leeyup, C. (2015). A Brief Introduction to the Transduction of Neural Activity into Fos Signal. Development & Reproduction, 19(2): 61-67. doi: 10.12717/DR.2015.19.2.061
Naso, M., Tomkowicz, B., Perry, W., and Strohl, W. (2017). Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs, 31: 317-334. doi: 10.1007/s40259-017-0234-5
Deisseroth, K. and Hegemann, P. (2017). The form and function of channelrhodopsin. Science, 357(6356). doi: 10.1126/science.aan5544
Eylam, S. and Spector, A. (2003). Oral Amiloride Treatment Decreases Taste Sensitivity to Sodium Salts in C57BL/6J and DBA/2J Mice. Chemical Senses, 28(5): 447-458
Ossebaard, C., Polet, I., Smith, D. (1996). Amiloride Effects on Taste Quality: Comparison of Single and Multiple Response Category Procedures. Chemical Senses, 22(3): 267-275