Special K – Not just a cereal: ketamine as a novel treatment of depression
Traditional antidepressants have delayed therapeutic onset and are ineffective in up to 30% of treatment resistant forms of depression. Ketamine may provide a novel fast acting mechanism of reversing depression by changing the structure and signaling in the brain.
Author: Daniel Salazar
Download: [ PDF ]
Neurophysiology
It is not yet fully understood how ketamine, a commonly used anesthetic, reverses depression and anxiety symptoms. Moda-Sava and colleagues recently published a paper in Science Journals, aiming to understand how chronic stress affects the connectivity of the brain, neuronal structure, and behavior in mice. Strikingly, they discovered that ketamine is able to reverse depression symptoms and improve connectivity of the brain within 4 hours. After 24 hours, ketamine induces the regrowth and addition of structural components of neurons. Furthermore, they observed that this regrowth and addition of new structural components of neurons attributed to the sustainable remission of depression behavior in mice. This research provides application of ketamine as a powerful clinical tool in treating depression and sustaining recovery.
DEPRESSION AND KETAMINE
Depression is a complex mental disorder which results from a combination of genetic and environmental factors. Growing research has shown that depression may arise from misfiring neurons.1 Specifically, the excitatory neurons in areas of the brain important in executive function and memory formation.1 This is formally known as the “Glutamate Hypothesis” of depression. Depression is also associated with loss of dendritic spines.2 Dendritic spines are tiny protrusions coming off of neurons. Additionally, another hallmark of depression is chronic stress.3 Thus, scientists use stress-induced-depression rodent models to understand the underlying mechanisms of depression and to develop future treatments. Unfortunately, up to 30% of people who suffer from depression do not respond to traditional treatments such as SSRIs and cognitive behavioral therapy.3,4 Moreover, traditional antidepressants often take weeks to take effect.4 Severe symptoms of depression include suicidal ideation and self harm. Thus, therapeutic onset of antidepressant medication is extremely important in these circumstances.5 In humans, ketamine has shown promise in acutely reversing depression symptoms within 24 hours.6 However, the way in which ketamine acts on the brain to reverse depression is not yet fully understood.
METHODS
Moda-sava and colleagues aimed to understand the role of ketamine on depression-like behavior in mice. They monitored apical spine density in the mPFC before and after chronic stress exposure as well as after intraperitoneal ketamine injections. Chronic stress was applied with 21 days of corticosterone administration in the drinking water OR 21 days of restrained stress test. These researchers were able to monitor the structural changes in dendritic spines using microprisms and optic fibers implanted in the mPFC of the mice. Two photon microscopy was used to image the dendrites before and after exposure to chronic stress and ketamine administration. Additionally, connectivity within mPFC microcircuits was measured using 2 photon calcium imaging. Behavioral tests included sucrose preference test, exploration of the open arms in the elevated plus maze, and mobility during the tail suspension test.
KETAMINE REVERSES DEPRESSION INDUCED BEHAVIOR AND CHANGES IN THE BRAIN
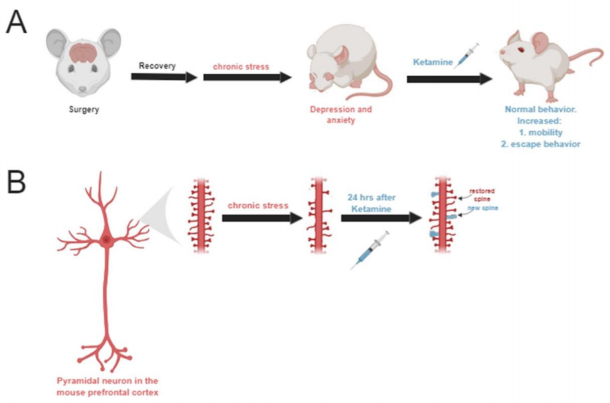
Unsurprisingly, 21 days of chronic stress led to the development of depression and anxiety-like behavior in mice. Two photon microscopy revealed that dendritic spine density had also decreased following chronic stress. Remarkably, ketamine administration reversed depressionlike behavior within 4 hours after the injections (Figure A). Connectivity and synapse formation also improved within the mPFC within 4 hours after ketamine. Interestingly, there was no significant changes in spine densities until after 24 hours post ketamine injections. At this 24 hour mark, they saw an increase in dendritic spine re-growth as well as the addition of new spines (Figure B). Next, Moda-Sava and his team wanted to test whether ketamine provoked stochastic or spine specific regrowth/addition. They discovered that deletion of new spines led to relapse in behavior and deletion of random spines had no significant effect on behavior.
SIGNIFICANCE AND FUTURE STUDIES
These findings suggests that spinogenesis plays a vital role in the sustained recovery of depression. Study author Dr. Liston states, "Our results suggest that interventions aimed at enhancing synapse formation and prolonging their survival could be useful for maintaining the antidepressant effects of ketamine in the days and weeks after treatment." Future studies should focus on developing treatments that prolong the life of synapses in conjunction to ketamine treatment. Other glutamatergic system targeted therapies should also be further explored in the future. Ketamine may serve as a powerful clinical tool in treating depression and importantly in elucidating the complexities underlying mental illness.
[+] References
Sanacora, G., Treccani, G., & Popoli, M. (2012, January). Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21827775
Qiao, H., An, S., Xu, C., & Ma, X. (2017, May 15). Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28284898
Seo, J., Wei, J., Qin, L., Kim, Y., Yan, Z., & Greengard, P. (2017, October). Cellular and molecular basis for stress-induced depression. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27457815
Al-Harbi, K. S. (2012). Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/
Health Quality Ontario. (2017, November 13). Psychotherapy for Major Depressive Disorder and Generalized Anxiety Disorder: A Health Technology Assessment. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5709536/
Lener, M. S., Kadriu, B., & Zarate, C. A. (2017, March). Ketamine and Beyond: Investigations into the Potential of Glutamatergic Agents to Treat Depression. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5342919/