Why it doesn’t hurt to get enough sleep
Researchers find that even subtle day-to-day changes in sleep patterns can significantly alter the perception of pain.
Author: Cheyanne Lewis
Download: [ PDF ]
Neurophysiology
The relationship between sleep and pain can be a vicious cycle – what happens as a problem in one area leads to problems in the other.1 Those who must deal with chronic or acute pain report fragmentations in their sleep cycle such that they are unexpectedly woken up in the middle of the night. These fragmentations, referred to as microarousals, are recurrent brief awakenings, often fifteen seconds in duration, during which a person will suddenly move from deep sleep to wakefulness.2 These microarousals are not always linked to pain, however, as they are associated with a variety of conditions including elevated blood pressure, high cholesterol, and stress.3 Yet, several studies have found that even if microarousals are not caused by pain, they can ultimately lead to pain.4,5 More specifically, they can alter pain sensitivity.
Following repeated sleep disturbance, there is not only a reduction in duration, but in sleep quality. There is a loss in the most important phase of sleep: rapid eye movement (REM) sleep. Different from the stages of non-REM sleep, REM sleep is considered paradoxical in that physiological changes are similar to a person’s waking state with increases in breathing and heart rate and, of course, rapid eye movements. The chemical and electrical imbalances associated with REM brain wave activity are believed to have origin within the brain stem following large electrical bursts of energy. REM sleep is most often associated with memory formation and learning, and in losing precious REM sleep, there is the result of sleep debt. Sleep debt is what follows extended periods of wakefulness and occurs when the stages of sleep cannot fully cycle throughout the night.6 There is a biological “cost” that accumulates, and sometimes the result is hyperalgesia7,8: an increased sensitivity to pain.
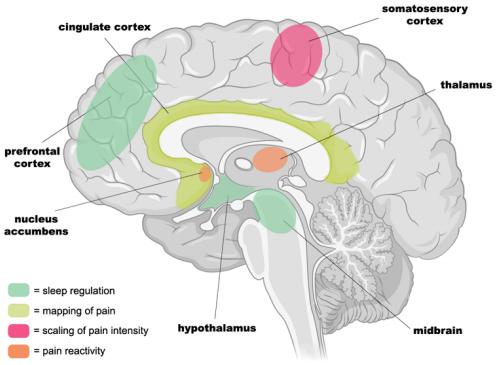
Specific neural networks within the brain are responsible for pain intensity as well as encoding higher-order evaluative processes following painful stimuli (Figure 1). Together, these regions constitute the pain-reactivity network. Activity within the somatosensory cortex creates a scale as a function of pain intensity that increases in increments between no detectable pain and maximum pain threshold.9 The cingulate cortex as well as the insula are responsible for the higher order processing of internal state changes in response to pain10 while the nucleus accumbens encodes for emotional value and saliency.11 Both of which are important for pain modulation. Changes in activity of these regions are hypothesized to account for increases in pain that follow sleep deprivation.
One recent study published in the Journal of Neuroscience explored the bidirectionality of sleep and pain to find out whether minor changes in sleep, in addition to full deprivation, can have significant effects on pain sensitivity. Krause and colleagues looked at changes within the painreactivity network by (1) measuring brain activity following acute sleep deprivation in a lab setting, and (2) using an online study following typical nightly changes within the general population.
The laboratory study involved 25 healthy participants who were free of sleep-related disorders, chronic pain, and injuries. Prior to the first session of the experiment, each participant underwent a quantitative sensory test to measure baseline thermal sensitivity between a temperature considered “warm, not yet painful” and one considered “painfully hot”. This step was particularly important to establish each participant’s thermal pain scale and ensure that observations of neural changes caused by sleep deprivation were not due to subjective differences in the experience of pain in response to objectively different thermal temperatures. During the sleep deprivation session, participants arrived at the laboratory late at night and were monitored while participating in low-stress activities such as internet surfing, reading, and movies of low emotionality. In the sleep-rested session, participants were prepared for an 8-hour night of sleep. At around 8:30 A.M. for both sessions, participants performed the thermal pain sensitivity task in the fMRI scanner to observe alterations in reactivity and if their perceived level of pain had changed. In the online phase of the study, a separate group of 236 participants were assessed over 2 nights and 2 days following their normal sleeping schedule. Throughout this phase, they quantified a number of factors related to sleep by filling out a sleep diary. Following this, they completed a questionnaire at the end of the day to rate their physical pain experience on a scale of 0-100 with 100 signifying unimaginable pain.
The researchers hypothesized that following sleep loss, activity within the somatosensory cortex, if directly correlated, would increase in scale with the degree of perceived pain. Decreases in activation would occur in the thalamus and nucleus accumbens assuming the threshold of pain had shifted, indicating a failure to modulate pain. Decreases in activity of the insula and cingulate would indicate improper integration of higher-order evaluation. And after analyzing the fMRI data, this is exactly what they had observed for the in-laboratory phase of the study. For the online portion, they found that subtle changes in sleep in the general population was a predictor of dayto-day changes in self-reported pain intensity in that insufficient sleep in terms of efficiency and quality correlated with higher ratings of experienced pain.
Together, these findings suggest that…
(1) acute sleep deprivation alters the range for classifying a stimulus as painful by lowering thresholds
(2) sleep loss alters the pain-reactivity network by increasing pain reactivity and halting higher order processes that are in charge of valuing the painful stimulus
(3) the same effects can be seen following both acute sleep deprivation and minor changes in sleep
This study is important in offering a new avenue for pain treatment, suggesting that modest improvements in sleep quality have the potential to significantly reduce feelings of pain. For many people, small changes that ensure quality, rather than quantity, are more realistic. Even if there isn’t enough time in the day to get the full recommended hours of sleep, finding ways to ensure quality, particularly in REM sleep, will at least have some protective effects against the dangerous cycle of pain and sleep loss.
[+] References
Anderson M.L, Araujo P., Frange C. & Tufik S. (2018) Sleep Disturbance and Pain: A Tale of Two Common Problems, Chest, 154(5): 1249-1259
Martin S.E., Englemen H.M., Kingshott R.N. & Douglas N.J. (1997) Microarousals in patients with sleep apnoea/hypopnoea syndrome, Journal of Sleep Research, 6: 276-280
Ekstedt M., Åkerstedt T. & Söderström M. (2004) Microarousals During Sleep Are Associated With Increased Levels of Lipids, Cortisol, and Blood Pressure, Psychomatic Medicine, 66(6): 925-931
.Krause A.J., Prather A.A., Wager T.D., Linquist M.A. & Walker M.P. (2019) The Pain of Sleep Loss: A Brain Characterization in Humans, The Jounral of Neuroscience, 39(12):2291-2300
Smith M.T., Haythornthwaite J.A. (2004) How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature, Sleep Medicine Reviews, 8(2): 119-132
Desseilles M., Dang-Vu T., Schwartz S., Peigneux P. & Maquet P. (2009) “Neuroimaging in Sleep and Sleep Disorders”. Sleep Disorders Medicine (Third Edition). Philadelphia, PA: W.B. Saunders
Roehrs T., Hyde M., Blaisdell B., Greenwald M. & Roth T. (2006) Sleep Loss and REM Loss are Hyperalgesic, Sleep, 29(2): 145-151
Lautenbacher S., Kundermann B. & Krieg J.C. (2005) Sleep deprivation and pain perception, Sleep Medicine Reviews, 10(5): 357-369
Bushnell M.C., Duncan G.H., Hofbauer R.K, Ha B., Chen J.I. & Carrier B. (1999) Pain perception: Is there a role for primary somatosensory cortex?, PNAS, 96(14): 7705-7709
Vogt B.A. & Sikes R.W. (2000) The medial pain system, cingulate cortex, and parallel processing of nociceptive information, The Biological Basis for Mind Body Interactions, 122: 223-235
Baliki M.N., Geha P.Y., Fields H.L. & Apkarian A.V. (2010) Predicting Value of Pain and Analgesia: Nucleus Accumbens Response to Noxious Stimuli Changes in the Presence of Chronic Pain, Neuron, 66(1): 149-160