Amyloid-𝛃 betta get outta here
A study found that local injection of gene editing proteins significantly improved the commonly observed neuroanatomical and behavioral changes associated with Alzheimer’s disease. Moreover, these findings advance the field of research investigating gene therapy as a novel method of treatment for neurodegenerative diseases.
Author: Jordan Donaldson
Download: [ PDF ]
Neurophysiology
Every 66 seconds someone is diagnosed with Alzheimer’s disease.1 Alzheimer’s is a disease which causes degeneration of the brain through a complex process where plaques accumulate within the brain causing memory dysfunction amongst other deficits, leading to death in many cases.2 Furthermore, it is estimated that by 2050, diagnoses will increase to every 33 seconds.1 Currently, Alzheimer’s disease is the 6th most common cause of death and has already deaths caused by stroke, heart disease, and prostate cancer combined.1 This results in over $236 billion dollars a year in health care fees.1 These statistics express the necessity for rapid development of treatments for Alzheimer’s disease. Fortunately, Hanseul Park and colleagues published in Nature Neuroscience this year investigating how gene therapy may be a treatment for people with Alzheimer’s disease. They found that their novel delivery technique of gene editing proteins significantly decreased toxic levels of the proteins clumps associated with Alzheimer’s disease. These findings suggest a new delivery method to the brain for many neurodegenerative diseases, resulting in more effective and precise prevention or treatment.
Alzheimer's disease is an irreversible neurodegenerative disease resulting in memory dysfunction and hippocampal degeneration due to the accumulation of plaques called 𝛃-amyloid plaques and tau tangles, amongst other etiologies.3,4,5 These 𝛃-amyloid plaques accumulate after the metabolism of 𝛃-amyloid precursor protein (APP) by 𝞬- and 𝛃-secretases6 (Figure 1). Therefore, targeting 𝛃-secretases activity serves as an important therapeutic target for preventing the accumulation of 𝛃-amyloid. Although 𝛃-amyloid accumulation does not account for the entirety of the neurodegenerative properties of Alzheimer’s disease, it has been shown to be one of the greatest contributions to the neurodegeneration.7 Current complications with developing treatments is delivery to the brain. This is because the vascular system of the brain forms what is known as the blood-brain-barrier (BBB).8 The BBB selectively allows compounds into the internal structures of the brain. Many drugs are unable to pass the BBB and therefore, treatments, no matter how effective in cell culture, are not effective in vivo.8 Thus, recent studies have been testing novel methods of treatment delivery across the BBB.
The study most recently published has explored the efficacy of amphiphilic nanoparticles. These nanoparticles contain the gene editing proteins collectively known as the amphiphilic-CRISPR-Cas9 construct or nanoparticles for short2 (Figure 1). An amphiphilic molecule is one that has a side of the membrane which is water soluble and the other side of the membrane is water insoluble, meaning that the particle is able to pass through water-insoluble membranes while holding water soluble components within the vesicle9 (Figure 1). Hanseul utilized the properties of the amphiphilic nanoparticles to deliver his drug across the BBB. The nanoparticle he developed contained a CRISPR Cas9 construct which targeted beta-secretase 1 (Bace1) and silenced the gene6,10,11 (Figure 1). The nanoparticles were injected into the hippocampus of mice which were genetically modified to reflect the pathophysiology of Alzheimer’s disease.2 This method of administration allowed for direct administration of the construct in a controlled and localized manner. Through use of these novel methods, they found astonishing results.
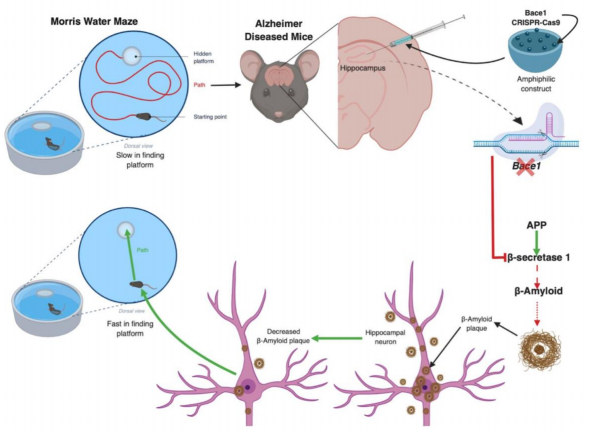
Upon injection of Bace1-Cas9 construct into the hippocampus they reported a 70% reduction of Bace1 expression.2 Then, they wanted to evaluate the phenotypic result from the reduction of Bace1. Hanseul Park had the mice perform various memory tasks such as the water maze and spontaneous alternation Y-maze test which both demonstrated a significant improvement in memory and learning in the nanoparticle treated mice compared to the Alzheimer’s diseased mice (Figure 1). These results are extremely important and clinically relevant as the mice only had to receive one low dose injection with significant reduction in both the plaque accumulation and memory deficits. Although these results are very reassuring for the advance of CRISPR-Cas9 applications, there are remaining concerns yet to be completely evaluated.
One of the concerns by the field is that there will be chronic off-site binding of the CRISPR-Cas9 system which would cause random mutations with unknown effects. Park addressed this concern in his paper by using whole-genome sequencing, whole-exome sequencing and Digenome sequencing and found that there was a low percent difference between the sham and nanoparticle treated mice.2 This thorough examination reassures immediate concerns, however, there have yet to be studies on the chronic effects of the system. Although Park reassured the reader by a study he performed, where he found that the Cas9 system was undetectable in the brain three weeks post-injection.2 Lastly, after the injection there was no observed increase in inflammation, cell death or microglia recruitment.2
In conclusion, this work greatly advances the reality of using gene therapy as a possible treatment for neurodegenerative disorders. As Park stated, the novel delivery system may also be used to treat localized neurodegenerative disorders. This opens the future field of research not only to the capabilities at attenuating the effects of Alzheimer's disease but also other diseases such as Parkinson’s disease. This study greatly increases the likelihood of remediation of this extremely serious and prevalent disease. Future studies will likely evaluate the effects in higher-order mammals prior to human trials.
[+] References
Alzheimers Dement “Alzheimer’s disease facts and figures.” Alzheimer’s Association. 2016; 124: 459–509
Park, Hanseul, et al. "In vivo neuronal gene editing via CRISPR–Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer’s disease." Nature neuroscience (2019): 1.
Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. β-Secretase Protein and Activity Are Increased in the Neocortex in Alzheimer Disease. Arch Neurol. 2002;59(9):1381–1389.
Serrano-Pozo, Alberto, et al. "Neuropathological alterations in Alzheimer disease." Cold Spring Harbor perspectives in medicine 1.1 (2011): a006189
Yang, Li-Bang, et al. "Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease." Nature medicine 9.1 (2003): 3.
Zilai Wang, Li Yang and Hui Zheng, “ Role of APP and Aβ in Synaptic Physiology”, Current Alzheimer Research (2012) 9: 217
Murphy, M Paul, and Harry LeVine 3rd. “Alzheimer's disease and the amyloid-beta peptide.” Journal of Alzheimer's disease : JAD vol. 19,1 (2010): 311-23
Pardridge, William M. “Drug transport across the blood-brain barrier.” Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism vol. 32,11 (2012): 1959-72. doi:10.1038/jcbfm.2012.126
Dehsorkhi, Ashkan et al. “Self-assembling amphiphilic peptides.” Journal of peptide science : an official publication of the European Peptide Society vol. 20,7 (2014): 453-67
Kang, E. L., Biscaro, B., Piazza, F., & Tesco, G. (2012). BACE1 Protein Endocytosis and Trafficking Are Differentially Regulated by Ubiquitination at Lysine 501 and the Di-leucine Motif in the Carboxyl Terminus. Journal of Biological Chemistry, 287(51), 42867–42880. doi:10.1074/jbc.m112.407072
Steven L. Roberds, John Anderson, Guriqbal Basi, Michael J. Bienkowski, Daniel G. Branstetter, Karen S. Chen, Stephen Freedman, Normand L. Frigon, Dora Games, Kang Hu, Kelly Johnson-Wood, Karl E. Kappenman, Thomas T. Kawabe, Ismail Kola, Ralf Kuehn, Michael Lee, Weiqun Liu, Ruth Motter, Nanette F. Nichols, Michael Power, David W. Robertson, Dale Schenk, Michael Schoor, George M. Shopp, Mary E. Shuck, Sukanto Sinha, Kjell A. Svensson, Gwen Tatsuno, Hartmut Tintrup, John Wijsman, Sarah Wright, Lisa McConlogue, “BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: implications for Alzheimer’s disease therapeutics”, Human Molecular Genetics, Volume 10, Issue 12, 1 June 2001, Pages 1317–1324