Deep brain stimulation improves cognitive control in depressed patients
Author: Maxwell Neideigh
Download: [ PDF ]
Neurophysiology
New research suggests that increasing “theta” rhythms, frequencies in the brain easily observed in EEG recordings, provides neurosurgeons and psychiatrists with the consistent, and reliable feedback needed to calibrate the placement and intensity of Deep Brain Stimulation. Deep brain stimulation is a method used by neurosurgeons to treat a myriad of neurodegenerative diseases, that involves the placement of stimulating electrodes to specific brain regions. The greatest success thus far in the field has been in treating Parkinson’s disease, where patients under the influence of DBS experience an overall reduction in the amount of tremors they would normally have as a result of the disease. Present day research has extended the reach of DBS not only as a treatment for tremors, but as a therapy to treat psychiatric disorders like major depressive disorder (MDD) and obsessive compulsive disorder (OCD).
Assistant professor of psychiatry at the University of Minnesota Medical School Alike Widge explains that, one way to alleviate symptoms associated with depression is to induce synchronized Theta brain wave activity in a brain region called the ventral striatum.1 In rats, theta wave rhythmicity can be observed in the hippocampus, but can also be seen in a number of other cortical and subcortical brain structures. Ventral Striatum theta waves carry a frequency with a range of 6–10 Hz, and are predominantly seen when a rat is engaged in locomotion like walking back and forth in its cage, when a new smell enters its nose, and when the rat is in its deepest phase of the sleep cycle called REM sleep.2
Widge explains that both MDD and OCD patients are characterized as having a lack of cognitive control which is needed to interfere with negative thinking patterns and obsessive impulses.3 When it comes to MDD and OCD, evidence for patient improvement is much more subtle than in the case with Parkinson’s disease, where neurosurgeons can see a reduction in tremors almost immediately following calibration of the electrodes and stimulator.4 One hypothesis for how DBS can help depressive symptoms presented by Alik Widge, the lead and corresponding author on the paper, “Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function,” published on April 4th, involves reinforcing theta rhythms in the prefrontal cortex. DBS has been shown to have an impact on what Widge’s team of researchers would call “cognitive control,” an ability primarily attributed to the prefrontal cortex, and an ability most MDD and OCD patients struggle with.
In the April 4th paper published in Nature Communications researchers used 14 patients who have had previous DBS treatment for their depression. Each participant was faced with a background image displaying emotionally evocative content, and then were told to complete a conflict task involving a series of numbers. Participants brain waves were recorded during the conflict tasks once with DBS turned on and once with DBS turned off (Figure 1c). When Theta rhythms in the cortex increased as a result of DBS, depressed patients chose the correct answer to the conflict task faster and more accurately than when the DBS was absent.
Exploring further into the field of DBS neurosurgeons may discover more effective sites for stimulation to treat the symptoms associated with depression like habitual negative thinking by reinforcing theta brain wave activity and improving cognitive control. Obsessive compulsive disorder is another psychiatric disorder that is characterized by a person having uncontrollable, reoccurring thoughts or obsessions, that can be interfered with through DBS. Furthermore, DBS may provide patients who are chronically stuck in compulsive or negative thinking patterns an alternative to traditional antidepressant medication like serotonin specific reuptake inhibitors (SSRI’s), which are only slightly effective at treating these symptoms in the long term.5
Cognitive control tasks that use DBS typically involve participants viewing an emotionally arousing image while trying to pick numerical values in a sequence of three that doesn’t fit (Fig. 1a). The images purpose is to place an emotional “distractor” on to the patient, simulating a real world situation that requires cognitive control to overcome. Participants performed the cognitive control task under the influence of DBS and without it. Emotionally arousing images present distractors to the participants, inhibiting their ability to react quickly and accurately to the series of numbers.
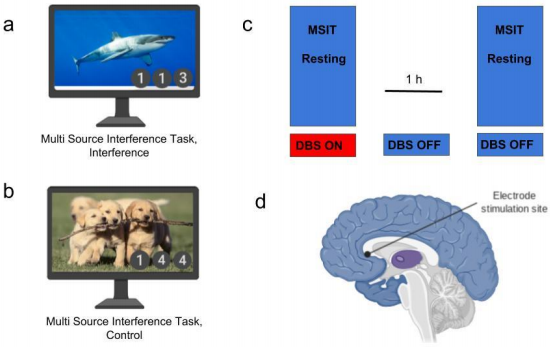
Deep brain stimulation improves performance on cognitive control tasks and increases theta oscillations in the medial, and lateral prefrontal cortex. With DBS on, subjects in-fact made their choice quicker demonstrating their ability to overcome the interference of the emotional picture, without sacrificing accuracy.
Previous studies indicate deep brain stimulation (DBS) has proven to be an effective therapeutic option for alleviating tremors associated with Parkinson’s Disease and now more recently, restoring cognitive control. The current study demonstrates that DBS to an FDA approved target for psychiatric disabilities, is shown to impact a particular symptom, that lies at the root of multiple psychiatric illnesses.
These findings suggest that DBS may provide patients who are chronically stuck in compulsive or negative thinking patterns an alternative to prescription medication to treat their symptoms. DBS therapies that focus on activating theta brain waves can reinforce particular suppressed neural networks that may be implicated in a range of diseases related to mental health.
[+] References
S. Widge, S. Zorowitz, I. Basu, A. C. Paulk, S. S. Cash, E. N. Eskandar, T. Deckersbach, E. K. Miller & D. D. Dougherty. Nature Communications volume 10, Article number: 1536 (2019) https://doi.org/10.1038/s41467-019-09557-4
Kirk IJ (1998). "Frequency modulation of hippocampal theta by the supramammillary nucleus, and other hypothalamo-hippocampal interactions: mechanisms and functional implications". Neurosci Biobehav Rev. 22(2): 291–302. https://doi.org/10.1016/S0149-7634(97)00015-8. PMID 9579319
Klimkeit, EI; Mattingley, JB; Sheppard, DM; Farrow, M; Bradshaw, JL (2004). "Examining the development of attention and executive functions in children with a novel paradigm". Child Neuropsychology. 10 (3): 201–211. doi:10.1080/09297040409609811. PMID 1559049
McIntyre CC, Thakor NV (2002). "Uncovering the mechanisms of deep brain stimulation for Parkinson's disease through functional imaging, neural recording, and neural modeling". Critical Reviews in Biomedical Engineering. 30 (4–6): 249–81. https://doi.org/10.1615/critrevbiomedeng.v30.i456.20
Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van Noord M, Mager U, Thieda P, Gaynes BN, Wilkins T, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN (December 2011). "Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis". Annals of Internal Medicine. 155 (11): 772–85. https://doi.org/10.7326/0003-4819-155-11-201112060-00009. PMID 22147715
Accolla EA, Aust S, Merkl A, Schneider GH, Kühn AA, Bajbouj M, Draganski B (April 2016). "Deep brain stimulation of the posterior gyrus rectus region for treatment resistant depression". Journal of Affective Disorders. 194: 33–7. https://doi.org/10.1016/j.jad.2016.01.022
Sainsbury, RS; Heynen A; Montoya CP (1987). "Behavioral correlates of hippocampal type 2 theta in the rat". Physiol Behav. 39 (4): 513–519. https://doi.org/10.1016/0031-9384(87)90382-9. PMID 3575499
Tauscher-Wisniewski S, Nilsson M, Caldwell C, Plewes J, Allen AJ (October 2007). "Meta-analysis of aggression and/or hostility-related events in children and adolescents treated with fluoxetine compared with placebo". Journal of Child and Adolescent Psychopharmacology. 17 (5): 713–8. https://doi.org/10.1089/cap.2006.0138. PMID 17979590
Volkmann J, Herzog J, Kopper F, Deuschl G (2002). "Introduction to the programming of deep brain stimulators". Movement Disorders. 17 Suppl 3: S181–7. doi:10.1002/mds.10162
Weaver, Frances M. (2009). "Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson DiseaseA Randomized Controlled Trial". JAMA. 301 (1): 63. doi:10.1001/jama.2008.929