The brain on cocaine: a closer look at the cellular changes to blame
New research gives insight into the cellular changes that occur during cocaine addiction, a meaningful step towards treating the disorder.
Author: Jonathan Anguiano
Download: [ PDF ]
Neurophysiology
Cocaine is a highly addictive drug and has the potential to ruin the lives of those who fall victim to its grasp. In 2017, roughly 966,000 individuals of age 12 or older suffered from a cocaine use disorder.1 Of this population, many will relapse after a period of abstinence. Those who undergo standard treatment programs for cocaine addiction are similarly prone to relapse. The root of cocaine’s evil lies in its power over the brain. If better treatments for cocaine addiction are to be developed, one must look to the brain and how cocaine influences its function. In a recent study published in eNeuro, Slaker et al.2 elucidate the mechanisms underlying cocaine addiction by investigating the cocaine-induced cellular changes that occur in a brain region known as the medial prefrontal cortex (mPFC). Slaker and her research team were interested in this brain region because of past research showing that the mPFC controls behaviors related to cocaine addiction, at least, in rodents.3 Additionally, Slaker et al. had conducted a previous study showing that removal of a structure known as the perineuronal net (PNN) in the mPFC could decrease relapse in a rodent model of cocaine addiction.4 Many studies have revealed PNNs to be involved in learning and memory.5,6 In light of this, Slaker et al. concluded from the study that removing PNNs disrupts a rodent’s memory of the rewards associated with cocaine, eliminating the drive for cocaine and decreasing relapse. The study described here builds on this research by characterizing the cellular changes in PNN-surrounded neurons. Interestingly, more than 90% of PNNs in the mPFC surround a specialized neuron known as the fast-spiking interneuron (FSI).2 FSIs contribute greatly to the function of the mPFC and are themselves involved in learning and memory.7,8 FSIs accomplish this via communication with principal neurons, which project to various brain regions to influence behavior. Important to the development of treatments for cocaine addiction, Slaker et al. reveal significant changes in FSIs and PNNs that may contribute to cocaine addiction and relapse.
To replicate cocaine addiction in humans, the researchers administered cocaine to rats over the course of five days. The researchers then assessed how cocaine impacted levels of excitatory and inhibitory input onto FSIs. In general, excitatory input activates a neuron, like stepping on the gas pedal to ramp up the speed of a car. On the other hand, inhibitory input suppresses a neuron, like slamming on the brakes to come to a halt. After five days of cocaine administration, FSIs display signs of increased inhibitory input and an increased responsiveness to inhibitory input. Slaker et al. also measured cocaine-induced changes in FSI electrical properties. FSIs are themselves inhibitory, and measuring such changes in electrical properties gives insight into how cocaine affects the inhibitory control of principal neurons and, therefore, behavior. Slaker et al. found that the FSIs were overall less excitable after cocaine administration, meaning their ability to inhibit would be greatly reduced. Additionally, the level of PNNs surrounding FSIs was decreased after one day of cocaine administration.
The study conducted by Slaker et al. represents an important advancement in the realm of understanding cocaine addiction. The results described above demonstrate that cocaine alters the excitatory/inhibitory balance. The excitatory/inhibitory balance describes an appropriate level of excitatory and inhibitory responses to a given event.9 When this balance is disrupted, disease states, such as that of addiction, can ensue. In the mPFC, cocaine appears to decrease the ability of FSIs to inhibit principal neurons. This decrease frees principal neurons from inhibitory control, increasing excitation elicited by principal neurons and possibly leading to relapse (Fig 1). Indeed, previous research has shown that principal neurons display increased excitability in response to repeated cocaine administration.10,11
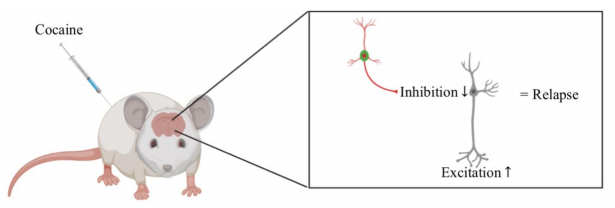
Slaker et al. further suggest that the changes in FSIs may contribute to the chronic nature of drug addiction by altering their function in communication networks.2 The impact of cocaine on FSI function may be in part mediated by the observed decrease in PNN levels. This is consistent with research showing that a specific component of PNNs, known as brevican, controls FSI function.12 While the research by Slaker et al. does not test treatments for cocaine addiction, it is a necessary first step in preventing and treating the disorder. The FSI and PNN system may one day be targeted in order to reverse or attenuate characteristics of cocaine addiction. For example, transcranial magnetic stimulation (TMS) is already used to alter excitation and inhibition in disorders such as major depressive disorder. If the precise mechanism of cocaine addiction in the mPFC are identified, TMS may be able to target and manipulate activity of said brain region and thereby reverse characteristics of the disorder. Accordingly, future research should attempt to determine how FSIs might be taken advantage of in treating cocaine addiction.
[+] References
Substance Abuse and Mental Health Services Administration. (2018). Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/
Slaker, M. L., Jorgensen, E. T., Hegarty, D. M., Liu, X., Kong, Y., Zhang, F., . . . Sorg, B. A. (2018). Cocaine Exposure Modulates Perineuronal Nets and Synaptic Excitability of Fast-Spiking Interneurons in the Medial Prefrontal Cortex. Eneuro 5. doi:10.1523/eneuro.0221-18.2018
McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine- seeking behavior in rats. Psychopharmacology 168:57–65.
Slaker, M., Churchill, L., Todd, R. P., Blacktop, J. M., Zuloaga, D. G., Raber, J., . . . Sorg, B. A. (2015). Removal of Perineuronal Nets in the Medial Prefrontal Cortex Impairs the Acquisition and Reconsolidation of a CocaineInduced Conditioned Place Preference Memory. Journal of Neuroscience 35:4190-4202. doi:10.1523/jneurosci.3592-14.2015
Balmer, T. S., Carels, V. M., Frisch, J. L., & Nick, T. A. (2009). Modulation of perineuronal nets and parvalbumin with developmental song learning. The Journal of Neuroscience 29:12878-85
Romberg, C., Yang, S., Melani, R., Andrews, M. R., Horner, A. E., Spillantini, M. G., Bussey, T. J., Fawcett, J. W., Pizzorusso, T., ... Saksida, L. M. (2013). Depletion of perineuronal nets enhances recognition memory and longterm depression in the perirhinal cortex. The Journal of Neuroscience 33:7057-65
Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL, Sohal VS (2014) Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron 81:61–68
Hu, H., Gan, J., & Jonas, P. (2014). Fast-spiking, parvalbumin GABAergic interneurons: From cellular design to microcircuit function. Science 345:1255263-1255263. doi:10.1126/science.1255263
Okun, M., & Lampl, I. (2009). Balance of excitation and inhibition. Scholarpedia, 4(8):7467
Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ (2005) Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci 25:936–940
Nasif FJ, Sidiropoulou K, Hu XT, White FJ (2005) Repeated cocaine administration increases membrane excitability of pyramidal neu- rons in the rat medial prefrontal cortex. J Pharmacol Exp Ther 312:1305–1313
Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B (2017) Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95:639– 655.e10.