Targeting chondroitin sulfate proteoglycans in order to reduce neural inflammation and promote oligodendrogenesis
Damage to the central nervous system results in limited tissue regeneration and permanent injuries such as paralysis. A study based in the University of Manitoba done by Dyck et al shows the cellular mechanism available for targeting to counteract this phenomenon.
Author: Jason Driver
Download: [ PDF ]
Neurophysiology
In 2007 the World Health Organization estimated the prevalence of those suffering from neurological disorders to be greater than 1 in 7, with more than 1 billion cases of neurological damage and 6.8 million deaths each year attributed to neurological damage.1 A unique feature about central nervous system damage is that it is generally viewed to be permanent. For the most part, damaged neural connections will remain in that state for the rest of the subject’s life.2 The motivation for this research was to better understand the reasons the body does not recover from spinal cord injury, with the long term hope of applying this knowledge towards building treatments.
The resistance of the adult CNS to regeneration is rooted in its physiology, specifically its extracellular matrix. The matrix inhibits regeneration through three classes of molecules; myelinassociated growth inhibitors, chemorepulsive guidance molecules, and highly sulfated proteoglycans with the greatest contributing factor being the proteoglycans often called CSPGs.3
It has been seen for centuries that the central nervous system resists regeneration, with spinal cord injuries and brain trauma being permanent.4 As analytical technology has been improved upon and new techniques and machines created, our ability to understand the why behind the central nervous system rejecting regeneration has also improved. The extracellular matrix of the central nervous system differs greatly from the majority of the body, including the peripheral nervous system. Oligodendrocytes and CNS associated astrocytes and microglia all excrete multiple forms of growth inhibiting factors, the most prominent of which being CSPGs.5 CSPGs consist of a protein core and a sugar chondroitin sulfate side chain; the sugar side chain sulfonation pattern can change influencing how CSPGs bind and the inhibitory factors associated with them. CSPGs inhibit neuroregeneration by binding to growth-inhibitory receptors found at axons, directly activating the growth inhibitory pathway, inhibiting neurotrophic receptors and activating phosphatases.6 Though, while it is known that CSPGs play an integral role in neural regulation, it is not fully understood how they do so.
One portion that is known, is that leukocyte common antigen-related (LAR), and protein tyrosine phosphatase-sigma (PTPσ) are both CSPG signalling receptors of oligodendrocytes in the CNS.7 It’s been seen that inhibition of PTPσ and LAR receptors promotes oligodendrogenesis, supporting recovery of the spinal cord.6
The article in focus from Dyck et al., 2019 goes into more depth on how the LAR PTPσ pathway can be targeted in a potentially clinically beneficial manner.8
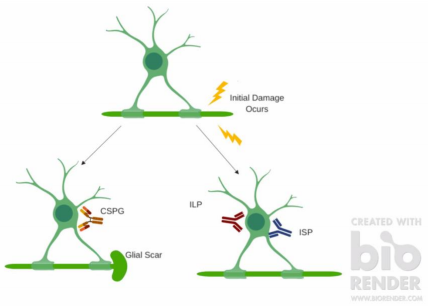
The study used female Sprague Dawley rats and targeted LAR and PTPσ through LAR peptide (ILP) and intracellular sigma peptide (ISP); two peptides blocking the receptors for LAR and PTPσ.9 ILP and ISP are designed to bind to a 24-amino acid intracellular wedge domain on oligodendrocytes, modulating the catalytic activity of these receptors. Rats had their spinal cords crushed and solutions of ILP and ISP solutions were injected at the injury site in order to attempt to change the body’s inflammatory and regenerative response. Figure 1 shows a cartoon model of the divergence in how the body addresses damage.
The study found a significant decrease in inflammation, pointing to the use of ILP & ISP solutions as an area of more study for recovering from spinal cord injury. The study specifically cited CSPG signaling manipulation, the basis of this research, as a promising approach to promote recovery. Overall, the work done by Dr. Dyck focusing on the microenvironment of spinal cord injuries is ground breaking and provides hope for the future of this research.
[+] References
Neurological disorders affect millions globally: WHO report. (2007, February 27). Retrieved March 18, 2019, from https://www.who.int/mediacentre/news/releases/2007/pr04/en/
Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. Recovery from Neural Injury. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10856/
Sharma, K., Selzer, M. E., & Li, S. (2012). Scar-mediated inhibition and CSPG receptors in the CNS. Experimental neurology, 237(2), 370-8.
Todd, R. B. (1855). Clinical lectures on paralysis, disease of the brain, and other affections of the nervous system. Lindsay & Blakiston.
Miller, G. M., & Hsieh-Wilson, L. C. (2015). Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Experimental neurology, 274( Pt B), 115-25.
Lutz, A. B., & Barres, B. A. (2014). Contrasting the glial response to axon injury in the central and peripheral nervous systems. Developmental cell, 28( 1), 7-17
Takahashi, H., & Craig, A. M. (2013). Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization. Trends in neurosciences, 36(9), 522–534. doi:10.1016/j.tins.2013.06.002
Dyck, S., Kataria, H., Akbari-Kelachayeh, K., Silver, J., & Karimi-Abdolrezaee, S. (2019). LAR and PTPσ receptors are negative regulators of oligodendrogenesis and oligodendrocyte integrity in spinal cord injury. Glia, 67(1), 125-145
Properzi, F., Asher, R. A., & Fawcett, J. W. (2003). Chondroitin sulphate proteoglycans in the central nervous system: changes and synthesis after injury.
Xie, Y., Massa, S. M., Ensslen-Craig, S. E., Major, D. L., Yang, T., Tisi, M. A., ... & Brady-Kalnay, S. M. (2006). Protein-tyrosine Phosphatase (PTP) Wedge Domain Peptides A NOVEL APPROACH FOR INHIBITION OF PTP FUNCTION AND AUGMENTATION OF PROTEIN-TYROSINE KINASE FUNCTION. Journal of Biological Chemistry, 281( 24), 16482-16492
Dyck, S., Kataria, H., Alizadeh, A., Santhosh, K. T., Lang, B., Silver, J., & Karimi-Abdolrezaee, S. (2018). Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury. Journal of neuroinflammation, 15( 1), 90. doi:10.1186/s12974- 018-1128-2
Dyck, S. M., & Karimi-Abdolrezaee, S. (2018). Role of chondroitin sulfate proteoglycan signaling in regulating neuroinflammation following spinal cord injury. Neural regeneration research, 13( 12), 2080–2082. doi:10.4103/1673-5374.241452
Lau, L. W., Keough, M. B., Haylock-Jacobs, S., Cua, R., Döring, A., Sloka, S., ... & Yong, V. W. (2012). Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Annals ofneurology, 72(3), 419- 432
[+] Other Work By Jason Driver
The unique properties between the cerebral cortex and sports-related concussive injuries
Neuroanatomy
Concussions are a large issue with a growing spotlight in sports; professionally, collegiately, and recreationally this is a problem commonly addressed. Maerlender et al’s article “Concussion competencies: a training model for school-based concussion management” chose to take a look at how concussive education can decrease risky behavior and overall cortical damage.