The role of the alpha 7 nicotinic acetylcholine receptor subunit in temporal processing, inflammation, and learning
Author: Vicente Chavez
Download: [ PDF ]
Neuroscience In Review
Introduction: alpha 7 subunit role in learning
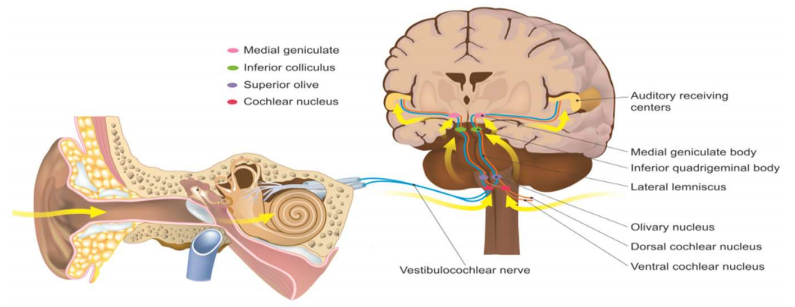
Learning is a multifaceted mechanism that integrates a breadth of modules from our environments such as touch, vision, and sound. Complex sounds such as speech and music are all processed by different nuclei throughout the auditory pathway, traveling from the periphery where sound is captured to the auditory cortex for interpretation (Figure 1). Disruptions throughout the transmission pathway may result in a broad range of auditory processing disorders, many associated with neurodevelopmental disorders such as Autism Spectrum Disorder (ASD) or Asperger’s syndrome.1 Detection of ASD continues to increase in response to clearer diagnostic technology and procedures.2 Research suggests a link between these pervasive developmental disorders and auditory processing disorders that impede one’s ability to form speech and in turn face communication obstacles.3 School aged children receive clinical tests that are not able to detect neuropathies such as central auditory processing disorders.3 Tests include raising one’s hand in response to a noise and detecting which ear a noice came out of. These children may experience long term developmental complications in academia and social life.3 A group significantly affected by these identification gaps include people with ASD. Brain regions implicated in ASD also are involved in auditory processing and memory, both important for auditory learning. Many neurodevelopmental disorders such as ASD have an underlying dysregulation of inflammation.4 A promising pharmaceutical target for these inflammatory regulation includes the nicotinic acetylcholine receptor because of its ubiquitous roles in the peripheral and central nervous systems as an immunomodulator. Specifically, alpha 7 subunit acetylcholine receptors serve a vital role in our cholinergic anti-inflammatory pathway and can lead to neurodegenerative diseases if immunomodulatory function is chronically disrupted.5 I emphasize the role of alpha 7 subunit (alpha 7 nAChR) in inflammation, central auditory processing disorders (APDs), and auditory learning. Current research on these subunits and their relationship with inflammation and auditory processing focuses on treatments to reduce inflammation and receptor regulation to increase auditory processing and increase learning outcomes.
Alpha 7 nAChR knockout decreases auditory processing precision
Auditory processing is complex and neural networks responsible for processing sound begin developing before birth.7 Processing complex sounds relies heavily on precise timing of sounds in our environment for proper encoding to occur and small millisecond changes can distort perception.8 Hearing loss in children can lead to disrupted speech production and learning difficulties.7 The auditory cortex neural projections of normal-hearing children and those born with congenital deafness have distinct observable differences. If children are born with hearing loss or other neurodegenerative disorders that effect auditory processing, then those brain regions affected undergo structural plasticity.9 However, if auditory processing dysfunction is identified early on before critical periods of development, then treatment such as cochlear implants can be applied in hopes of preserving the auditory cortex neural projections.9,10
Nicotinic acetylcholine receptors are abundant throughout the central and peripheral nervous system and important in neurodevelopmental signaling.11 Specifically, the alpha 7 nAChRs in the central nervous system are of interest because of their role in auditory learning. Research has identified degraded temporal processing of auditory information in the midbrain and brainstem in alpha 7 knockout mice.12 This degraded signaling can result in social and cognitive challenges for developing children.13 The effected nuclei include the inferior colliculus (IC), superior paraolivary nucleus (SPON), and ventral nucleus of the lateral lemniscus (VNLL).13 The IC has an important role in auditory integration encoding semantics of complex sounds. Previous studies have linked alpha 7 KO mice with maturation delays potentially due to high levls of alpha 7 expression in early development in the VNLL which send signals to the IC.14 The mechanism that inhibits precise timing is unknown, but a clear link between alpha 7 nAChRs and timing precision exists. Precise temporal processing results in proper speech formation and early age disruptions can result in learning impairments.
Alpha 7 mediated cholinergic anti-inflammatory pathway
Our immune system plays a vital role in the background of all biological processes. The body is a war zone and the immune system is the military. The frontline soldiers include macrophages and microglia primed to engage with all internal and external threats with an arsenal of cytokines. Cytokines induce different immune responses such as pain and inflammation. Tight inflammatory regulation exists maintaining homeostasis between pro-inflammatory and anti-inflammatory agents. Inflammation dysregulation in the central and peripheral nervous system is associated with a wide range of neurodegenerative disorders, most notably ASDs, multiple sclerosis, and Alzheimer’s Disease.15,16
Studies into the inflammatory mechanism behind macrophages have found alpha 7 nAChRs as the key inflammatory mediator.17 Findings demonstrated mice without alpha 7 receptors failed to inhibit macrophage tumor necrosis factor (TNF) release upon vagal nerve stimulation.17 Since these findings numerous studies have confirmed the alpha 7 presence in macrophages and has inspired research into alpha 7 agonist as anti-inflammatory agents. Alpha 7 receptors in macrophage serving an immunomodulatory function provided a template for the search of a central nervous system receptor in microglial cells and isolated alpha 7 nAChRs to also mediate similar inflammatory pathways.5 Further investigations found microgial cells to have the ability to modulate TNF release evoked by lipopolysaccharides through activation of alpha 7 nAChR.5 Central APDs can be a result of inflammatory lesions due to chronic inflammation.18
Binaural fusion occurs at the pons effectively integrating information received from both ear pathways that are encoded at the IC and SPON. This intricate and delicate integration of auditory information has been implicated in spatial detection deficits as a result of brainstem lesions inducing chronic inflammation.19 The endogenous mechanisms are not well understood, but alpha 7 nAChRs serve as a promising pharmaceutical target to treat inflammation.
Future treatments and conclusion
Control of the cholinergic anti-inflammatory pathway present in the central and peripheral nervous system is a promising pharmaceutical target for anti-inflammatory control. It is clear that alpha 7 nAChRs are main modulators in the cholinergic anti-inflammatory pathway, but the underlying mechanisms are not well understood.20 Conflicting studies have results that are inconsistent with nicotinic binding and sometimes contradict what has been observed in vivo.20 For example, ulcerative colitis with nicotine or other nicotinic ligands have mixed results. Also, nicotine has a relatively low selectivity for a variety of nicotinic receptor subtypes confounding contributions and results of alpha 7.20 This a potential result of nicotine synergistic signaling because nicotine has a relatively low selectively for a variety of nicotinic receptors, a highly selective ligand should be explored to test efficacy.20
Similarly, to technology made for heart pacemakers, exploration of vagus nerve stimulators to attenuate macrophage mediated inflammation is warranted.21 Vagal nerve stimulation has been found to mediate microglia morphological changes that may provide insight into the underlying mechanism by further investigating the signaling cascade that leads to these changes. In conclusion alpha 7 nAChRs serve a central role in auditory processing and the anti-inflammatory pathway in macrophages and microglia. This may result in inflammatory lesions resulting in APDs inhibiting learning. Further research into the underlying mechanism of microglia antiinflammatory pathway are needed.
[+] References
O'Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev. 2012;36(2):836- 854. doi:10.1016/j.neubiorev.2011.11.008
Autism Spectrum Disorder. (2018). Retrieved August 15, 2020, from https://www.nimh.nih.gov/health/topics/autism spectrum-disorders-asd/index.shtml
Eggermont, J. J. (2015). Auditory temporal processing and its disorders. OUP Oxford.
Rossignol, D. A., & Frye, R. E. (2014). Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Frontiers in physiology, 5, 150.
Shytle, R. D., Mori, T., Townsend, K., Vendrame, M., Sun, N., Zeng, J., ... & Tan, J. (2004). Cholinergic modulation of microglial activation by α7 nicotinic receptors. Journal of neurochemistry, 89(2), 337-343.
The Auditory Pathway. (n.d.). Retrieved August 15, 2020, from https://teachmeanatomy.info/neuroanatomy/pathways/auditory-pathway/
Gordon, K. A., Papsin, B. C., & Harrison, R. V. (2003). Activity-dependent developmental plasticity of the auditory brain stem in children who use cochlear implants. Ear and hearing, 24(6), 485-500.
Eggermont, J. J. (2001). Between sound and perception: reviewing the search for a neural code. Hearing research, 157(1-2), 1-42.
Syka, J. (2002). Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiological reviews, 82(3), 601-636
Skoe, E., Chandrasekaran, B., Spitzer, E. R., Wong, P. C., & Kraus, N. (2014). Human brainstem plasticity: the interaction of stimulus probability and auditory learning. Neurobiology of learning and memory, 109, 82-93.
Morley, B. J., & Mervis, R. F. (2013). Dendritic spine alterations in the hippocampus and parietal cortex of alpha7 nicotinic acetylcholine receptor knockout mice. Neuroscience, 233, 54-63.
Felix 2nd, R. A., Chavez, V. A., Novicio, D. M., Morley, B. J., & Portfors, C. V. (2019). Nicotinic acetylcholine receptor subunit α7-knockout mice exhibit degraded auditory temporal processing. Journal of neurophysiology, 122(2), 451-465
Bailey, T. (2010). Auditory pathways and processes: implications for neuropsychological assessment and diagnosis of children and adolescents. Child Neuropsychology, 16(6), 521-548.
Baumann, V. J., & Koch, U. (2017). Perinatal nicotine exposure impairs the maturation of glutamatergic inputs in the auditory brainstem. The Journal of physiology, 595(11), 3573-3590
Depino, A. M. (2013). Peripheral and central inflammation in autism spectrum disorders. Molecular and Cellular Neuroscience, 53, 69-76
Conejero-Goldberg, C., Davies, P., & Ulloa, L. (2008). Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neuroscience & Biobehavioral Reviews, 32(4), 693-706
Wang H., Yu M., Ochani M. et al. (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388
Merrill, J. E., & Benveniste, E. N. (1996). Cytokines in inflammatory brain lesions: helpful and harmful. Trends in neurosciences, 19(8), 331-338
Griffiths, T. D., Bates, D., Rees, A., Witton, C., Gholkar, A., & Green, G. G. (1997). Sound movement detection deficit due to a brainstem lesion. Journal of Neurology, Neurosurgery & Psychiatry, 62(5), 522-526
Bencherif, M., Lippiello, P. M., Lucas, R., & Marrero, M. B. (2011). Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cellular and Molecular Life Sciences, 68(6), 931-949
Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., Watkins, L. R., ... & Tracey, K. J. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature, 405(6785), 458-462
[+] Other Work By Vicente Chavez
Cannabis helps your brain perform like new
Neurophysiology
Chronic low doses of THC can restore your brain power and help you perform as if you were half your age.
Eat your stress away!
Neuroanatomy
Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice.