A new target for depression treatment?
It was found that overexpression of Regulator of G Protein Signaling 8 (RGS8) protein is linked to resistance of depression. This could lead to a new drug target for treatment of depression.
Author: Edward Hernandez
Download: [ PDF ]
Neuroanatomy
Introduction
In a recent study published in Neuroscience, researchers at Hiroshima University investigate a protein, known as Regulator of G Protein Signaling 8, involvement in depression.1 Depression is a mental disorder that affects many people. In 2015, there were around 215 million people worldwide diagnosed with depression.2 The Diagnostic and Statistical Manual of Mental Disorders’ criteria for depression diagnosis are various symptoms that include sad mood, fatigue, weight loss, or worthlessness for at least 2 weeks.3 The cause of depression is currently unknown, but it is thought to be caused through a variety of factors as proposed through the biopsychosocial model.4 Examples of these factors are genetics, psychological trauma, and other illnesses. One major biological cause of depression is thought to occur through the monoamine hypothesis. The monoamine hypothesis suggests the predominant physiological cause of depression is an inadequate function of monoamine neurotransmitters, such as serotonin and dopamine.5 However, this study found when mice with significantly increased RGS8 were compared to regular mice, the overexpression of RGS8 helped to promote resistance to depressive symptoms in the mice.1 This was discovered through a comparison in a forced swimming test, use of a monoamine antidepressant and use of an antagonist of MCHRI, a receptor involved in emotion processing and energy homeostasis and that is also affected by RGS8.1 This study suggests RGS8 and MCHRI are other physiological factors that have a relationship with depression without the involvement of monoamines. This could lead to new treatment targets for people who are resistant to drugs that target monoamines.
Background
G proteins are important in transmitting signals from the exterior of the cell to the interior. G proteins are activated through G protein-coupled receptors and inactivated through regulator of G protein Signaling (RGS) protein. RGS8 is a specific RGS that has previously been found to be densely populated in the brain and to be antagonistic to Melanin-concentrating hormone receptor 1 (MCHR1).6 Stimulation of the receptor, MCHR1, has been found to lead to depressive behaviors.7 Furthermore, antagonism of the MCHR1 receptor, results in anti-obesity and antidepressant effects.8 The theory is that increased RGS8 will inhibit MCHR1, reducing depressive behaviors.
Methods
In the study, mice were genetically modified to form transgenic mice with increased RGS8 (tmRGS8). These mice appeared healthy with no abnormal behaviors or anatomy. Biopsies of their tails were tested using PCR to confirm the presence of the gene for increased RGS8. An open field test was performed to measure the difference in spontaneous locomotion. A forced swimming test (FST) was performed with the mice to test for depressive behavior. The FST was performed by placing the mice in a cylinder (24 cm height x 17 cm diameter) full of water up to 13 cm, to force the mice to swim, for 6 min. The water was changed after every test. The mice were filmed while they were in the swim tank, and the total duration of immobility was then measured afterward. The mice were then tested on the FST, 1 hour after being given SNAP94847 (10mg/kg), a chemical used to inhibit MCHR1. They then were tested with Desipramine (3mg/kg), a tricyclic antidepressant that inhibits norepinephrine and serotonin, that was injected intraperitoneally before the test.1
Results
Immunohistochemistry staining for RGS8 was used to analyze the cerebellum and hippocampus, where the strongest signal of RGS8 was found by using in situ hybridization and RT-PCR. It was found that tmRGS8 mice had about 2.3x the amount of RGS8 expression compared to WT mice in the hippocampal region, CA1. RGS8 expression in both mice was similar in other areas.5 For the open field test, there were no significant differences found in spontaneous locomotion. However, the tmRGS8 mice did have a significantly lower immobility time when compared to the WT mice. When the FST was performed with SNAP94847, the immobility time for tmRGS8 did not significantly decrease, but it did significantly decrease with WT mice. When the FST was performed with Desipramine, the immobility time for tmRGS8 was further decreased, while the effect for the wild type mice was similar to the effect of SNAP94847 (Kobayashi, 2018).
Discussion
Based on the results of the study, it can be concluded that increased expression of RGS8 may cause decreased depressive symptoms. This results from RGS8 possibly inhibiting the activity of MCHR1. This was found as tmRGS8 mice decreased immobility time with a monoamine antidepressant, Desipramine, but did not with the MCHR1 antagonist, SNAP94847. This suggests that the increased RGS8 was already acting on MCHR1. For the WT mice, SNAP94847 and Desipramine had similar effects on decreasing immobility time. This suggests that treatment of RGS8 and MCHR1 may be as effective as a monoamine antidepressant. This is important for future research as most of the current medications for depression have been modeled to act after the monoamine theory.9 This is a problem because around 10-30% of people with major depression, do not improve with this antidepressant treatment and some even have a poorer life quality, as well as taking a few weeks to work adequately.10 It is also known that SSRIs may increase the risk of suicide in adolescents and are not as effective in treating depression.11 Further research into RGS8 and MCHR1 may result in new treatment discoveries that may be as effective as monoamine antidepressants and may help those that are resistant to current treatments.
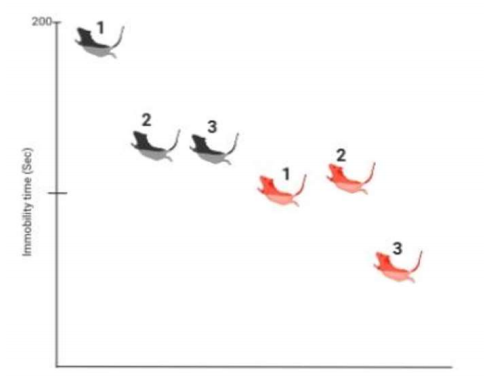
Effects of desipramine and SNAP94847 on the immobility time of mice. The black mice (BM) represent wild type mice, and the red mice (RM) represent tmRGS8 mice. 1= no treatment, 2= treatment with SNAP94847, 3= treatment with desipramine. All mice had significantly less immobility than BM1. RM3 had significantly less immobility than RM1, but RM2 did not.
[+] References
Kobayashi, Y., Takemoto, R., Yamato, S., Okada, T., Iijima, M., Uematsu, Y., Chaki, S., Saito, Y. (2018) Depression-resistant Phenotype in Mice Overexpressing Regulator of G Protein Signaling 8 (RGS8). Neuroscience, 383: 160 doi: 10.1016/j.neuroscience.2018.05.00.
GBD Disease and Injury Incidence and Prevalence Collaborators (2015) "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–602. https://doi.org/10.1016/S0140-6736(16)31678-6.
American Psychiatric Association (2013), “Diagnostic and Statistical Manual of Mental Disorders (5th ed.)”, Arlington: American Psychiatric Publishing, pp. 160–68, ISBN 978-0-89042-555-8.
Department of Health and Human Services (1999). "The fundamentals of mental health and mental illness" Mental Health: A Report of the Surgeon General.
Ruhé HG, Mason NS, Schene AH (2007). "Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies". Molecular Psychiatry, 12 (4): 331–59. doi:10.1038/sj.mp.4001949.
M. Miyamoto-Matsubara, O. Saitoh, K. Maruyama, Y. Aizaki, Y. Saito (2008) Regulation of melanin-concentrating hormone receptor 1 signaling by RGS8 with the receptor third intracellular loop. Cell Signal, 20, pp. 2084-2094.
Lagos, P., Urbanavicius, J., Scorza, M.C., Miraballes, R., Torterolo, P., (2011) Depressive-like profile induced by MCH microinjections into the dorsal raphe nucleus evaluated in the forced swim test. Behav Brain Res, 218, pp. 259- 266.
Gehlert, D.R., Rasmussen, K., Shaw, J., Li, X., Ardayfio, P., Craft, L., Coskun, T., Zhang, H.Y., Chen, Y., Witkin, J.M., (2009) Preclinical evaluation of melanin-concentrating hormone receptor 1 antagonism for the treatment of obesity and depression”. J Pharmacol Exp Ther, 329, pp. 429-438.
Boku, S., Nakagawa, S., Toda, H. and Hishimoto, A. (2018), Neural basis of major depressive disorder: Beyond monoamine hypothesis”. Psychiatry Clin. Neurosci., 72: 3-12. doi:10.1111/pcn.12604.
Al‐Harbi, KS., (2012) Treatment‐resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence; 6: 369– 388.
Al‐Harbi, KS., (2012) Treatment‐resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence; 6: 369– 388.
Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN (2012). "Newer generation antidepressants for depressive disorders in children and adolescents". The Cochrane Database of Systematic Reviews. 11: CD004851. https://doi.org/10.1002/14651858.CD004851.pub3.
[+] Other Work By Edward Hernandez
Diagnosing Autism Spectrum Disorder: There’s an App for that!
Neurophysiology
Researchers study the potential to use a digital app on a phone as a screening tool to assist in the diagnosis of Autism Spectrum Disorder (ASD).