No lonelier reverse transcriptase inhibitor
In a paper written by Kang, et al., a host of newly discovered azide compounds have been implicated in the treatment of HIV/AIDS in humans. The compounds are demonstrated to interact with a hydrophobic channel as well as the non-nucleoside inhibitory binding pocket (NNIBP) which is also known at the allosteric site of the channel.
Author: Micah Black
Download: [ PDF ]
Neurophysiology
The Human immunodeficiency virus has infected a total of more than 37.9 million people worldwide in 2018, including more than 1.7 million newly infected people in 2018.1 It is still considered a pandemic, and the United Nations has created a joint program in an attempt to combat the disease (UNAIDS). Kang et al. have published a research paper regarding the topic of compounds that are designed to treat the virus.2 They are referred to as antiretroviral drugs and a large portion of this research study was devoted to drugs that inhibit the reverse transcriptase protein from reproducing the virus in living organisms.
One of the more promising avenues of research is the study of the HIV-1 reverse transcriptase protein. Many anti-HIV drugs seek to block the channel pore or to allosterically hinder access to the pore in order to reduce the possibility for the virus to reproduce. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are a class of compounds that are used to treat HIV, and some are implicated in their ability to specifically target the HIV-1 reverse transcriptase site.3,4 This class of compounds is referred to as HIV-1 NNRTIs. UNAIDS has stated that “62% of people living with HIV were receiving antiretroviral treatment in 2018” and approximately 50% of the compounds used in this treatment falls under the category of HIV-1 NNRTI.1 They bind themselves to the non-nucleoside inhibitory binding pocket (NNIBP) and serve to allosterically hinder the reverse transcriptase site, by inducing a conformational change in the protein and therefore preventing the DNA polymerase from functioning in such a way that it can proliferate the virus.5,6
Drug research often involves the creation of a ‘library’ of compounds that can all be used in a similar fashion to achieve a certain result. In the case of this study, a library of diverse azide substituents was created and they were labeled N1-N39. Three types of alkynes, labeled A1-A6 were combined with the azide substituents. An example of an NNRTI created in this study would be A1N1, followed by A1N2, and so on. The alkyne compounds and azide substituents were coupled together by employing a method of click chemistry derived from the use of copper in a process called copper(I)-catalyzed azide-alkyne (3+2) dipolar cycloaddition (CuAAC). This method boasts fast reaction time, high yields, little or no need for purification steps as well as nearcomplete chemoselectivity.7,8,9 CuAAC has undergone some recent developments in the field of chemistry and is rapidly becoming a primary method for generating a bioactive molecules library for research purposes. Following the testing of the synthesized NNRTI compounds, A3N19 has been considered one of the more promising compounds for the treatment of HIV.
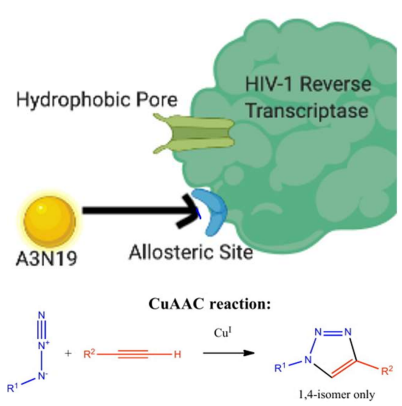
A list of each compound can be found in the research article, along with some of the reaction steps required to create alkyne starting materials A1-A6 along with their subsequent reactions with the azide starting materials. Click chemistry is a method through which a scientist can perform chemical reactions in physiological environments to demonstrate the function of organelles and other biological markers. There are many avenues that a researcher can take in order to study how chemicals can eradicate a virus and prevent it from spreading. This team’s avenue of research was to study how a triazole molecule can work to prevent a channel pore on the protein that is directly responsible for its replication may be the most viable method. Many years of research were required in order to understand the 3-dimensional conformation of the channel pore in question, as well as its conformational shape under a variety of physiological conditions.
[+] References
Data and statistics. (2020, July 09). Retrieved July 24, 2020, from https://www.who.int/hiv/data/en/
D. Kang, D. Feng, L. Jing, Y. Sun, F. Wei, X. Jiang, G. Wu, E. Clercq, C. Pannecouque, P. Zhan, X. Liu. “In Situ Click Chemistry-Based Rapid Discovery of Novel HIV-1 NNRTIs by Exploiting the Hydrophobic Channel and Tolerant Regions of NNIBP”. Elsevier European Journal of Medicinal Chemistry 193 (2020)
P. Zhan, C. Pannecouque, E. De Clercq, X. Liu, Anti-HIV drug discovery and development: current innovations and future trends, J. Med. Chem. 59 (2016)
D. Kang, T. Zhao, Z. Wang, D. Feng, H. Zhang, B. Huang, G. Wu, F. Wei, Z. Zhou, L. Jing, X. Zuo, Y. Tian, V. Poongavanam, J. Kongsted, E. De Clercq, C. Pannecouque, P. Zhan, X. Liu, Discovery of piperidine-substituted thiazolo [5,4-d]pyrimidine derivatives as potent and orally bioavailable HIV-1 non- nucleoside reverse transcriptase inhibitors, Commun. Chem. 2 (2019) 74.
D. Kang, H. Zhang, Z. Wang, T. Zhao, T. Ginex, F.J. Luque, Y. Yang, G. Wu, D. Feng, F. Wei, J. Zhang, E. De Clercq, C. Pannecouque, C.H. Chen, K.H. Lee, N.A. Murugan, T.A. Steitz, P. Zhan, X. Liu, Identification of dihydrofuro[3,4-d] pyrimidine derivatives as novel HIV-1 non-nucleoside reverse transcriptase inhibitors with promising antiviral activities and desirable physicochemical properties, J. Med. Chem. 62 (2019) 1484e1501.
G. Bec, B. Meyer, M.A. Gerard, J. Steger, K. Fauster, P. Wolff, D. Burnouf, R. Micura, P. Dumas, E. Ennifar, Thermodynamics of HIV-1 reverse transcriptase in action elucidates the mechanism of action of non-nucleoside inhibitors, J. Am. Chem. Soc. 135 (2013) 9743e9752.
X. Wang, B. Huang, X. Liu, P. Zhan, Discovery of bioactive molecules from CuAAC click-chemistry-based combinatorial libraries, Drug Discov. Today 21 (2016) 118e132.
T. Suzuki, Y. Ota, M. Ri, M. Bando, A. Gotoh, Y. Itoh, H. Tsumoto, P.R. Tatum, T. Mizukami, H. Nakagawa, S. Iida, R. Ueda, K. Shirahige, N. Miyata, Rapid discovery of highly potent and selective inhibitors of histone deacetylase 8 using click chemistry to generate candidate libraries, J. Med. Chem. 55 (2012) 9562e9575.
A.T. Carmona, S. Carrion-Jimenez, V. Pingitore, E. Moreno-Clavijo, I. Robina, A.J. Moreno-Vargas, Harnessing pyrrolidine iminosugars into dimeric struc- tures for the rapid discovery of divalent glycosidase inhibitors, Eur. J. Med. Chem. 151 (2018) 765e776.
X. Jiang, X. Hao, L. Jing, G. Wu, D. Kang, X. Liu, P. Zhan, Recent applications of click chemistry in drug discovery, Expet Opin. Drug Discov. 14 (2019) 779e789.
Kang, D., Zhang, H., Zhou, Z., Huang, B., Naesens, L., Zhan, P., & Liu, X. (2016). First discovery of novel 3-hydroxy-quinazoline-2,4(1H,3H)-diones as specific anti-vaccinia and adenovirus agents via 'privileged scaffold' refining approach. Bioorganic & medicinal chemistry letters, 26(21), 5182–5186.
L. Jing, G. Wu, X. Hao, F.A. Olotu, D. Kang, C.H. Chen, K.H. Lee, M.E.S. Soliman, X. Liu, Y. Song, P. Zhan, Identification of highly potent and selective Cdc 25 protein phosphatases inhibitors from miniaturization clickchemistry-based combinatorial libraries, Eur. J. Med. Chem. 183 (2019) 111696.
[+] Other Work By Micah Black
TRPA1: A Ghost ship in the membrane
Neuroanatomy
Transient Receptor Potential Ankyrin 1 is a calcium-permeable channel that plays a major role in pain perception, overactivity of these cation channels can induce hyperalgesia. A number of compounds belonging to various chemical families that were involved in animal/human studies have been demonstrated to act as antagonists to this TRP class of channel proteins and attenuate symptoms of hyperalgesia.