Gene therapy for epilepsy
Snowball et. al have potentially discovered a way to bypass invasive surgeries commonly used to treat epilepsy, often risking a patient’s ability to function normally. This new method utilizes a less invasive gene therapy approach targeting the KCNA1 potassium channel gene, in turn decreasing the disorder’s effect.
Author: Jessica Faraca
Download: [ PDF ]
Neurophysiology
An article published by Snowball et al. in The Journal of Neuroscience investigated potential ways of treating refractory focal epilepsy using gene therapy techniques.1 Refractory focal epilepsy is a form of epilepsy which is resistant to antiepileptic medications.2 Currently, treatments for epilepsy mainly consist of medication or surgery. Of those diagnosed with epilepsy, 30-40% do not respond to treatment with medication.3 This leaves a large percentage of the epileptic population considering surgery, and not every patient will be a viable surgical candidate. Surgical interventions can also pose risks to function and may result in severe side effects.4 Researchers found when implanting a gene encoding modified voltage-gated potassium channels via a viral vector there was a significant reduction in seizures in a rat model of focal neocortical epilepsy.1 These findings suggest gene therapy techniques are a potential noninvasive option for treating refractory focal epilepsy.
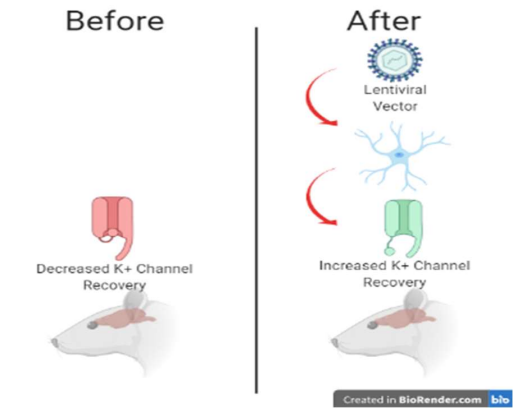
Snowball et al. began by engineering the Kv1.1 voltage-gated potassium channel gene, KCNA1, to accelerate the recovery time following inactivation.1 By increasing the recovery time of voltage-gated potassium channels the idea is hyperpolarization will be reached more successfully. This would then decrease the cells’ likelihood of firing again in rapid succession as seen in epilepsy.5 In order to incorporate the engineered potassium channel gene, the team of researchers utilized a nonintegrating lentiviral vector. Lentiviral vectors are a form of viral vector derived from human immunodeficiency virus (HIV).6 These viral vectors allow for genetic information to be incorporated into the host cell. Snowball et al. employed a non-integrating lentiviral vector, the non-integrating vector does not incorporate into the DNA of the individual, making it a safer option. The viral vector was under the control of a cell type-specific CAMK2A promoter.1 The animal model used in this research was a male rat for focal neocortical epilepsy. In order to quantify and analyze seizure reduction, continuous electrocorticography (ECoG) readings were taken. ECoG recordings measure the cortical potentials, it is often used to determine the location of epileptic tissues and predicted a surgery’s effectiveness in treating epilepsy.7 Seizure activity was then compared to baseline in order to determine the gene therapy’s effectiveness.
The team of researchers ended up finding a significant reduction in seizure activity compared to baseline in a male rat model of neocortical epilepsy. Following the lentiviruses injection, there was a decrease in seizure activity compared to control. The results of this preclinical trial suggest the engineered KCNA1 gene administered by a non-integrating lentiviral vector is a promising treatment for refractory focal epilepsy and further research should be done assessing its efficacy.
The team of researchers ended up finding a significant reduction in seizure activity compared to baseline in a male rat model of neocortical epilepsy. Following the lentiviruses injection, there was a decrease in seizure activity compared to control. The results of this preclinical trial suggest the engineered KCNA1 gene administered by a non-integrating lentiviral vector is a promising treatment for refractory focal epilepsy and further research should be done assessing its efficacy.
One thing to consider when considering the results of this research is the use of an animal model for refractory focal epilepsy. In humans there can be many different causes of epilepsy. And although mutated potassium channels are of great interest, there are many variations and phenotypes that may be possible.8 This study focused on the Kv1.1 voltagegated potassium channel and engineering of the KCNA1 gene. However, more studies will need to be completed in order to truly determine this specific gene therapy’s effectiveness. With that being said, the work performed by Snowball et al. is promising for the future of refractory focal epilepsy treatment and has the potential for clinical applications.
[+] References
Snowball, Albert, et al. “Epilepsy Gene Therapy Using an Engineered Potassium Channel.” The Journal of Neuroscience, vol. 39, no. 16, 2019, pp. 3159–3169., doi:10.1523/jneurosci.1143-18.2019.
Beleza P: Refractory Epilepsy: A Clinically Oriented Review. Eur Neurol 2009; 62:65-71. doi: 10.1159/000222775.
Engel J., Jr (2014). Approaches to refractory epilepsy. Annals of Indian Academy of Neurology, 17(Suppl 1), S12–S17. https://doi.org/10.4103/0972-2327.128644.
Engel J., Jr (2016). What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology, 87(23), 2483–2489. https://doi.org/10.1212/WNL.0000000000003407.
D'Adamo, M. C., Catacuzzeno, L., Di Giovanni, G., Franciolini, F., & Pessia, M. (2013). K(+) channelepsy: progress in the neurobiology of potassium channels and epilepsy. Frontiers in cellular neuroscience, 7, 134. https://doi.org/10.3389/fncel.2013.00134.
Milone, M. C., & O'Doherty, U. (2018). Clinical use of lentiviral vectors. Leukemia, 32(7), 1529–1541. https://doi.org/10.1038/s41375-018-0106-0.
DL Keene, S. Whiting, ECG Ventureyra. Electrocorticography. Epileptic Disorders. 2000; 2 (1): 57-64.
Kullmann, D., Schorge, S., Walker, M. et al. Gene therapy in epilepsy—is it time for clinical trials? Nat Rev Neurol 10, 300–304 (2014). https://doi.org/10.1038/nrneurol.2014.43.
[+] Other Work By Jessica Faraca
What makes us human? Newly discovered neurons may be the key
Neuroanatomy
Boldog et. al. have discovered a specialized GABAergic interneuron subtype within layer 1 cortex unique to the human brain. These rosehip interneurons have been shown to target layer 3 pyramidal neurons and work to inhibit back propagating, expressing great local control of distal dendrites
The neurological mechanisms of mindfulness meditation
Neuroscience In Review