Degeneration of cells stimulated by a-synuclein
The brain is not the only organ involved in the development of Parkinson's disease. Parkinson's disease spotted in the gut en route to the brain.
Author: Elisei Cosovan
Download: [ PDF ]
Neurophysiology
A neuronal pathologic protein, namely asynuclein, spreads from the gastrointestinal tract to the brain via the vagus nerve – eliciting similar features to those seen in Parkinson’s Disease (PD). This protein specifically stimulates the degeneration of the cells principal to PD-like motor and nonmotor symptoms. These cells are known as dopamine neurons. Surgical removal of part of the vagus nerve and a lack of the pathologic protein in this region resulted in the prevention of neurodegeneration and down-regulation of behavioral deficits in the PD mice models.1
The human gut microbiome has significant indirect effects on the brain. The diversity and health of the microbes in the gut also impact brain health in many ways. The gut microbiome is able to influence REM sleep, memory, mental health, and mood. The gut also has relevance in various disorders. Among these disorders are fibromyalgia, alcoholism, and chronic fatigue syndrome. The bacteria of the gut are able to directly stimulate afferent neurons to send signals to the brain. This occurs through the vagus nerve.
A large nerve that innervates much of the enteric nervous system, which will be referred to as the gut, but one which also reaches all the way up into the central nervous system. Overall, the gut uses this nerve as transportation. The bacteria stimulates a mechanism that travels to the cells in the brain which then sends signals to the rest of the body to perform a specific function.2 The gut microbes have a say in stress activity and sleep performance as well. In this review, we will focus on the effect a certain pathologic protein has on PD.
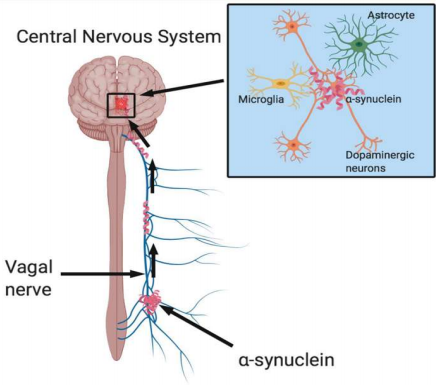
Parkinson’s Disease affects more than 200,000 people in the United States annually and yet no cure has been discovered for this disease.3 A study done by Sangjune Kim and a team of scientists, which was recently published in Neuron, examined the effects of gut-injected pathologic proteins on the brain in several groups of mice. The results of this study reveal that the pathologic protein, a-synuclein not only causes PD but also develops in the gut and is then transmitted to the brain via the vagus nerve pathway (Fig. 1).1 It is also discovered that dopamine neurons – which are lost as PD develops – are degenerated in mouse models containing the pathologic protein transmitted from the gut to the bran. The study reviewed here serves to fast-track research on specific cellular and molecular pathways related to the centripetal trafficking of the pathologic proteins in the route from the gut to the brain. For future research, this study will also aid in testing possible therapeutic interventions that will diminish the risk of PD.
The pathology of this protein follows a similar pattern of transmission through the body as the Lewy bodies observed in dementia in postmortem brains. The Lewy bodies are composed of clumps of the a-syn protein and are transmitted from the dendrite to the axon in a retrograde manner.4,5,6 Accumulation of these proteins is one of the main characteristics of neurodegenerative diseases.7 Braak, a German scientist of the 20th century observed a-syn pathology spreading from the gut to the brain through the vagus nerve.8 This observation is tested in this study through mouse models.
PFF (preformed fibrils) injections were made into the muscularis layer of the pylorus and duodenum – so to mimic the spread of a-syn in PD.9 The pylorus is the opening of the stomach which leads into the small intestine (also known as the duodenum). Together, these two structures will be termed as the gut throughout this review. The reason behind injecting PFF in this part of the gut is due to the high density of vagus nerve intervention in the area. Once the volume that could be injected between the muscles was determined, a set amount of 2.5 microliters was injected. A pSer129-a-syn immunostaining – which works as a stain that indicates a-syn – was used to observe the advancement of this protein from the gut to the brain. The progression of the protein was observed 1 month, 3 months, 7 months, then 10 months after the injection.
One month after the injection, the stained protein was detected in the medulla oblongata and the pons. These are two structures located in the upper and lower regions of the brainstem, respectively. The stain was also seen in the gut (specifically, the opening of the stomach and the small intestine). The protein was further observed accumulating in the brainstem 3 months after injection. Additionally, the staining indicated protein aggregation in the amygdala and in the ventral midbrain. The amygdala is a region of the brain located just above the brainstem, towards the center of the brain. Small amounts of protein were also seen in the hypothalamus and prefrontal cortex; two brain regions located deeper in the brain than the amygdala. Seven months after the injection the protein was observed more extensively in all regions mentioned earlier, plus in the hippocampus and the striatum. These two regions are deeper in the brain than the regions mentioned earlier. Finally, 10 months after injection, a-syn increased in the olfactory bulb, the hippocampus, the prefrontal cortex, the substantia nigra pars compacta, and the striatum. A decrease in protein accumulation was detected in the amygdala, the medulla oblongata, and the ventral midbrain. It was also concluded that there was a significant loss of dopamine neurons 3 months after the gut injection in the mice, then more drastically 7 months after injection. To test whether or not the vagus nerve is required for the transmission of the pathologic protein from the gut to the brain, part of the nerve was surgically removed. 7 months after the surgery, a 65% decrease in the amount of positive cholinergic neurons – which function to transmit messages – was observed. The pathologic proteins were still observed in some regions of the brain 7 months after the PFF injection, and partial removal of the nerve. When the vagus nerve was entirely removed, the spread of the pathologic protein was not observed. Upon behavioral analysis, it was concluded that the PFF-injected mice (with vagus nerve intact) had significantly decreased latency to fall by the rotarod test, and significantly increased the amount of time on the pole test. These tests display the key features observed in PD.
The results of this study support the Braak hypothesis and the notion that the pathologic protein, a-syn efficiently spreads from the gut to the brain via the vagus nerve. These results are consistent with Braak’s hypothesis because they offer a probable mechanism for the induction of a-syn pathology in the enteric nervous system.10,11,12 Additionally, these results support the idea that Parkinson’s Disease begins in the gut and transmits to the brain because the a-syn protein was seen in brain regions that are anatomically connected to each other. Although the results of this study imply that the transmission of pathological a-syn follows interneuronal transmission patterns, the Lewy body pathology was not observed in all affected brain regions.5 Further studies need to be done to differentiate which aspects of transmission contribute to the selective susceptibility of the given neuronal systems to the Lewy body pathology. Overall, I believe this study did a wonderful job of testing the Braak hypothesis and providing sufficient evidence to support the idea that PD begins in the gut and is then transmitted to the brain via the vagus nerve.
[+] References
Kim, S., Kwon, S. H., Kam, T. I., Panicker, N., Karuppagounder, S. S., Lee, S., ... & Shen, C. (2019). Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron, 103(4), 627-641.
Galland L. (2014). The gut microbiome and the brain. Journal of medicinal food, 17(12), 1261–1272. https://doi.org/10.1089/jmf.2014.7000.
Adams Jr, J., Chang, M. L., & Klaidman, L. (2001). Parkinsons Disease-redox mechanisms. Current medicinal chemistry, 8(7), 809-814.
Braak, H., Del Tredici, K., Ru€b, U., de Vos, R.A., Jansen Steur, E.N., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease.
Surmeier, D.J., Obeso, J.A., and Halliday, G.M. (2017). Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 18, 101–113.
Luk, K.C., Kehm, V., Carroll, J., Zhang, B., O’Brien, P., Trojanowski, J.Q., and Lee, V.M. (2012). Pathological asynuclein transmission initiates Parkinson- like neurodegeneration in nontransgenic mice. Science 338, 949–953.
Dodel, R., Csoti, I., Ebersbach, G., Fuchs, G., Hahne, M., Kuhn, W., Oechsner, M., Jost, W., Reichmann, H., and Schulz, J.B. (2008). Lewy body dementia and Parkinson’s disease with dementia. J. Neurol. 255 (Suppl 5), 39–47
Braak, H., Ghebremedhin, E., Ru€b, U., Bratzke, H., and Del Tredici, K. (2004). Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318, 121–134.
Berthoud, H.R., Carlon, N.R., and Powley, T.L. (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260, R200–R207.
Braak, H., Sastre, M., Bohl, J.R., de Vos, R.A., and Del Tredici, K. (2007). Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympa- thetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 113, 421–429.
Wakabayashi, K., Mori, F., Tanji, K., Orimo, S., and Takahashi, H. (2010). Involvement of the peripheral nervous system in synucleinopathies, tauopa- thies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol. 120, 1–12.
Kuo, Y.M., Li, Z., Jiao, Y., Gaborit, N., Pani, A.K., Orrison, B.M., Bruneau, B.G., Giasson, B.I., Smeyne, R.J., Gershon, M.D., and Nussbaum, R.L. (2010). Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 19, 1633–1650.
[+] Other Work By Elisei Cosovan
Try not to forget this one
Neuroanatomy
This study uses visual experiences and an instruction to disregard them in order to activate memory processing.
Alzheimer’s disease: detecting pathophysiological and neuropathological changes with biomarkers
Neuroscience In Review